Abstract
Histone acetylation and deacetylation play essential roles in eukaryotic gene regulation. Reversible modifications of core histones are catalyzed by two intrinsic enzymes, histone acetyltransferase and histone deacetylase (HD). In general, histone deacetylation is related to transcriptional gene silencing, whereas acetylation correlates with gene activation. We produced transgenic plants expressing the antisense Arabidopsis HD (AtHD1) gene. AtHD1 is a homolog of human HD1 and RPD3 global transcriptional regulator in yeast. Expression of the antisense AtHD1 caused dramatic reduction in endogenous AtHD1 transcription, resulting in accumulation of acetylated histones, notably tetraacetylated H4. Reduction in AtHD1 expression and AtHD1 production and changes in acetylation profiles were associated with various developmental abnormalities, including early senescence, ectopic expression of silenced genes, suppression of apical dominance, homeotic changes, heterochronic shift toward juvenility, flower defects, and male and female sterility. Some of the phenotypes could be attributed to ectopic expression of tissue-specific genes (e.g., SUPERMAN) in vegetative tissues. No changes in genomic DNA methylation were detected in the transgenic plants. These results suggest that AtHD1 is a global regulator, which controls gene expression during development through DNA-sequence independent or epigenetic mechanisms in plants. In addition to DNA methylation, histone modifications may be involved in a general regulatory mechanism responsible for plant plasticity and variation in nature.
Keywords: DNA methylation, epigenetics, gene silencing
Core histones can be acetylated or deacetylated through intrinsic activities of histone acetyltransferases or deacetylases (1). The acetylation state often relates to gene activity, whereas the deacetylation state is associated with inactivity (2). The deacetylation process involves the removal of acetyl moieties by deacetylases from specific lysine residues of core histones, thereby restoring positive charges on the lysine residues. The interaction between positively charged lysines and negatively charged DNA reduces nucleosome mobility on DNA, hindering accessibility of the promoter to the transcriptional machinery. Inhibition of histone deacetylase (HD) activity can reverse the process and result in gene activation. In eukaryotic organisms that use both DNA and histone modifications, HDs are recruited by DNA methyl-binding proteins (e.g., MeCP2, MBD2) (3–5), DNA methyltransferase (Dmnt1) (6), or sequence-specific DNA-binding proteins (7–10) to silence genes.
RPD3, an HD in yeast, is a global transcriptional regulator (11). RPD3-deletion mutants both up- and down-regulate gene expression in yeast (11–13) and enhance position-effect variegation in Drosophila (14). Mouse HD1, an RPD3 homolog, is identified as a growth factor-inducible gene (15). Overexpression of mouse HD1 in stable transfected mammalian cells causes a remarkable reduction in the growth rate and severe delay during the G2/M phases of the cell cycle, implying a role of histone acetylation in cell cycle progression.
Despite extensive studies on the role of histone modifications in eukaryotic gene regulation, the effects of histone deacetylation on plant gene regulation and development are unclear. It has been demonstrated that both histone deacetylation and DNA methylation are involved in silencing one parental set of rRNA genes in allotetraploid Brassica (16), a close relative of Arabidopsis. In this study, we used an antisense inhibition approach to down-regulate antisense Arabidopsis HD gene (AtHD1) expression. Antisense-AtHD1 transgenic plants had reduced levels of the HD and increased levels of tetraacetylated histone H4. As a result, these plants displayed ectopic expression of tissue-specific genes [e.g., SUPERMAN, (SUP)] (17) and various types of aberrant phenotypes. Some phenotypes were present in the subsequent selfing generations. Changes in DNA methylation in the repetitive and single-copy DNA sequences were not detected in the transgenic plants. We conclude that besides DNA methylation, histone deacetylation plays an essential role in plant gene regulation and development.
Materials and Methods
Cloning of HD Gene in Arabidopsis.
PCR was used to amplify AtHD1 fragments from total cDNA of Arabidopsis thaliana (Columbia) with the use of primers designed from human HD1 (18) (Fig. 1A). Sequences of three cDNA fragments matched an expressed sequence tag (G11C3T7) and AtHD1 (AF014824) in the Arabidopsis database. The insert was sequenced, and the expressed sequence tag clone was designated pAtHD1–2.
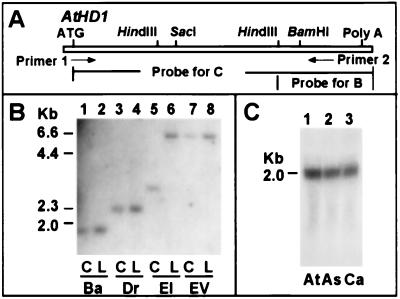
Figure 1.
AtHD1 is a single-copy gene and is expressed in Arabidopsis. (A) A simplified restriction map of AtHD1. Arrows indicate the primers used to amplify a 1.5-kb reverse transcription–PCR fragment. The fragments used as DNA probes are indicated below the diagram. (B) Autoradiogram showing a DNA blot containing genomic DNA from Columbia (C) and Landsberg (L), which was hybridized with the 3′ region (≈500 bp) of AtHD1 (A). Ba, BamHI; Dr, DraI; EI, EcoRI; EV, EcoRV. (C) An RNA blot was hybridized with a full-length cDNA fragment as a probe (A). At, A. thaliana, Ca, Cardaminopsis arenosa; As, A. suecica.
Production of Constitutive Antisense HD (CASH) Transgenic Plants.
A full-length cDNA fragment released from the pAtHD1–2 was ligated into the XbaI-KpnI sites of the pMON10098 vector (19). The expression of the antisense transgene is driven by a 35S cauliflower mosaic virus constitutive promoter. Agrobacterium-mediated transformation (20) was used to produce transgenic A. thaliana (Columbia). About 30,000 seeds (T0) were sterilized and germinated on Murashige/Skoog medium (Sigma) containing 50 mg/liter kanamycin. A total of 157 resistant seedlings (T1) were obtained and screened for the presence of the transgene by PCR. The primers were as follows: 35S: 5′-TGACGCACAATCCCACTATCCTTCGCA-3′; AtHD1-A3: 5′-CCTGATACAGAGACTCCCGAGGTTGAT-3′. Eleven homozygous CASH plants (T2 and T3) were further characterized in this study. Antisense DNA-methyltransferase (MET1) transgenic plants were also produced with the use of a full-length cDNA (21) and the same vector. The detailed characterization of 105 antisense-MET1 plants was omitted. One plant (no. 407) was used as a demethylation control in the DNA blot analysis. All plants were grown in vermiculite mixed with 10% soil in a growth chamber with growth conditions of 18/22°C (day/night) and 14 h of illumination per day. Photographs were taken with a Pentax (Lyndhurst, NJ) camera with a macro lens.
Nucleic Acid Isolation and Detection.
RNA and DNA were isolated from at least five leaves of each plant in the same developmental stages as previously described (16). Total RNA (20 μg) or DNA (2 μg) was subjected to electrophoresis in an agarose-formaldehyde or agarose gel and blotted onto Hybond-N+ membrane (Amersham Pharmacia). To make antisense or sense RNA probe, the plasmid pAtHD1–2 was linearized by digestion with XhoI or XbaI, respectively, and was used as a template in separate reactions to generate single-stranded RNA with in vitro synthesis kits (Promega). Sense and antisense AtHD1 was synthesized by the T3 and T7 RNA polymerases, respectively. The antisense SUP was synthesized with the use of SP6 RNA polymerase as previously described (17). The DNA probe was prepared by a random priming method. Hybridization was performed following the method of Church and Gilbert (22). The DNA and RNA blots were washed at 65°C in 2× SSC/0.2× SDS for 30–60 min (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7), and hybridization signals were detected by digital imaging analysis or exposure to Kodak x-ray film.
Antibody Production, Histone Isolation, and Immunoblotting.
The cDNA fragment containing the N terminus of AtHD1 (1–199 amino acids) was subcloned in frame in the pET21a expression vector (Novagen), which was then transformed into Escherichia coli (strain BL21). Recombinant AtHD1 protein was induced with isopropyl-β-d-thiogalactoside, purified by column chromatography, and sent to Cocalico Biologicals (Reamstown, PA) for polyclonal antibody production in rabbit. The antisera were used in 1:500 dilution for immunoblotting. The antibodies detected a major band (≈25 kDa) and a minor band (≈70 kDa) in recombinant protein (data not shown) but a single band (≈60 kDa, expected size) in crude protein extracts. The minor band detected in recombinant proteins may be caused by the presence of a small amount of nonspecific protein retained after histidine affinity purification. Antibodies against tetraacetylated histone H4 or nonacetylated histone H4 (H4) were purchased from Serotec and Upstate Biotechnology (Lake Placid, NY). Protein crude extracts and histone fractions were prepared as previously described (16) and subjected to electrophoresis in 8 and 15% SDS/PAGE, respectively. The immunoblots were probed with antisera AtHD1, tetraacetylated histone H4, and H4; antibody binding was then detected by enhanced chemiluminescence (Amersham Pharmacia).
Results
Characterization of HD Genes in Arabidopsis.
The nucleotide sequence of AtHD1 (AF014824) encodes 501 amino acids, with 56 and 55% amino acid sequence identity, respectively, to HD1 in mammals (18) and RPD3 in yeast (11). Thus, the Arabidopsis gene is named AtHD1. HD1 homologs are highly conserved with 55–96% overall identity among Arabidopsis, yeast (11), Drosophila (14), maize (23), and human (18).
The copy number of AtHD1 was examined by DNA blot analysis. Using the 3′ region of the cDNA fragment as a probe (Fig. 1A), we detected only single fragments in the genomic DNA digested with four restriction enzymes (Fig. 1B). Thus, AtHD1 is a single-copy gene. Genomic sequence of the gene is located on the short arm of chromosome 4 and tagged by the DNA marker mi390 (24). Another gene (HD2) has been identified in maize (25) and Arabidopsis (26). Two AtHD2 homologs (AtHD2A and AtHD2B) are primarily expressed in flowers and young siliques (26). Expression of AtHD1 (≈2 kb) was high in leaves in A. thaliana and its related species, Cardaminopsis arenosa and A. suecica (Fig. 1C).
Yeast has five related genes, RPD3, HDA1, HOS1, HOS2, and HOS3 (12), in addition to Sir2, that encode an NAD-dependent HD (27). On the basis of BLAST/N analysis, Arabidopsis has Sir2-like and other HD genes, including a single-copy AtHD1 or RPD3 (Fig. 1B), two copies of RPD3-related AtHDA1, at least two copies of AtHD2, and several homologs of HOS1, 2, and 3. In yeast, both RPD3 and HDA1 are purified (12). RPD3 and HDA1 have slightly different functions in deacetylating histones. RPD3 has greater effects than other homologs on deacetylating lysine residues 5 and 12 on histone H4 and plays a role in both heterochromatic gene silencing and inducible gene activation (11, 12).
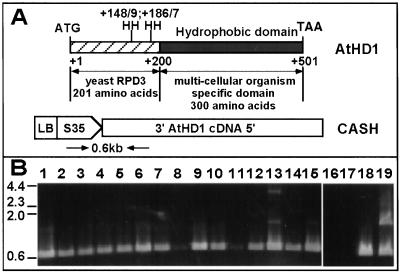
The AtHD1 has two components. The N terminus of the protein (201 amino acids) is homologous to yeast RPD3; the C terminus (300 amino acids) is highly hydrophobic and specific to multicellular eukaryotic organisms, including plants and mammals (Fig. 2A). The histidines at positions 148/149 and 186/187 are conserved catalytic sites for deacetylation activity in yeast (13).
Figure 2.
AtHD1 construct and transgene detection in transgenic plants. (A) The diagram of AtHD1 and CASH construct. N terminus of AtHD1 (201 amino acids) is homologous to yeast RPD3; the C terminus (300 amino acids) is highly hydrophobic and specific to multicellular organisms. The CASH construct is shown below the AtHD1 diagram. The primers used to amplify a ≈600-bp fragment in CASH plants are shown. (B) Ethidium bromide-stained agarose gel showing PCR-amplified fragments in CASH plants. Controls were a transgenic plant transformed with vector only (lane 16) and a CASH plant in a PCR reaction without primers (lane 17). More than one insert was present in some plants (lanes 13 and 19); low amplification was found in others (lanes 8 and 11).
Production of Antisense and Sense AtHD1 Transgenic Plants.
The antisense p35S∷AtHD1 construct and transgenics are referred to as CASH (Fig. 2A). The presence of the transgene in 157 CASH plants was screened by PCR amplification. A subset of the PCR results is shown in Fig. 2B. Of the 157 plants tested, 151 contained at least one insert; only 6 plants had no insert. Consistent with the PCR results, DNA blot analysis by using the kanamycin gene as a hybridization probe confirmed that all 50 plants analyzed have the transgene (data not shown). Eleven of them with one to three copies of the transgene were selected for further study.
Antisense AtHD1 Expression Down-Regulates Endogenous AtHD1 Expression.
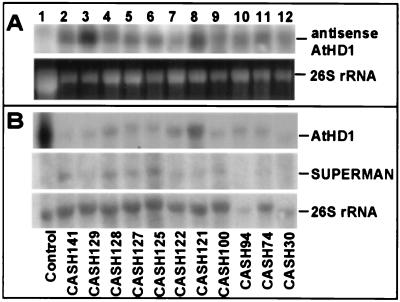
Expression of antisense AtHD1 transcripts was analyzed in CASH plants and a control plant (Fig. 3). Except as noted otherwise, “control” refers to plants transformed with the vector only. The antisense transcripts were highly expressed in 11 CASH plants (Fig. 3A, lanes 2–12) but were absent in the control (lane 1). As a result, endogenous AtHD1 transcripts were dramatically reduced, ranging from <5% (Fig. 3B, lanes 2, 3, and 12) to 40% (lane 8) of the level in the control (lane 1). Only trace amounts of AtHD1 transcripts were detected in three CASH plants (lanes 2, 3, and 12), most of which had severe phenotypes.
Figure 3.
Gene expression patterns in the control and CASH transgenic plants. Total RNA (20 μg) from the control and CASH plants was subjected to electrophoresis in a 1.2% agarose gel containing 2% formaldehyde and transferred onto Hybond-N+ membrane (Amersham Pharmacia). The blot was then hybridized with a radiolabeled probe with the use of either an in vitro transcript kit (for AtHD1 and SUP) or a random priming method (for 26S rDNA). (A) The membrane was probed with radiolabeled single-stranded RNA of sense AtHD1. An ethidium bromide-stained RNA gel was used as the RNA loading control. (B) The membrane was probed with radiolabeled single-stranded RNA of antisense AtHD1, antisense SUP, or 26S rDNA.
Although there was little variability in the levels of antisense AtHD1 overexpression (Fig. 3A), the levels of endogenous AtHD1 transcripts in the CASH plants varied from 5 to 40% of the level in the wild type (Fig. 3B). Gradient reductions in AtHD1 transcription in multiple independent CASH plants were correlated with stochastic effects of inhibiting AtHD1 expression on plant development (see below).
Ectopic Expression of SUPERMAN (SUP) in the CASH Transgenic Plants.
Normal control over gene expression was obviously disrupted in some CASH plants, in which ectopic expression of SUP (Fig. 3B, lanes 2, 4, and 6) and delay of flowering were frequently observed. One of the plants (CASH125) developed abnormal flowers without sepals and petals but with extra stamens. This phenotype is different from SUP, which has localized effects on floral whorl boundaries (17). It is likely that inhibiting histone deacetylation affects not only SUP expression but also expression of additional genes (e.g., B function genes) required for floral (sepal and petal) development. Weak expression of SUP was detected in three other CASH plants (lanes 5, 7, and 9), none of which developed SUP phenotypes. However, two of them (CASH100 and 127) developed homeotic changes, suggesting a role of histone deacetylation in plant development.
Down-Regulation of AtHD1 Induces Developmental Pleiotropy in Arabidopsis.
About 55% of the 151 multiple independent CASH plants showed visibly aberrant phenotypes (Table 1), including early senescence (9%), serration (17%), aerial rosette formation (23%), homeotic changes (4%), floral abnormalities (2%), and delay of flowering. A gradient of phenotypic changes existed in each category. It is notable that, with the exception of one plant that was transformed with vector only and displayed very weakly serrated leaves, none of the control or wild-type plants showed phenotypic abnormalities. This lack of abnormalities reduces the possibility that transformation artifacts were responsible for the observed phenotypic changes in the CASH plants.
Table 1.
Phenotypic variation in 151 independent transgenic plants containing CASH
| Genotypes: | Columbia
(CASH)
|
Columbia (vector)
|
Columbia
|
|||
|---|---|---|---|---|---|---|
| No. of plants, % | No. of plants, % | No. of plants, % | ||||
| Phenotypes* | ||||||
| 1. Early senescence | 14 | 9.2 | 0 | 0 | ||
| 2. Serrated leaves | 25 | 16.6 | 1† | 4 | 0 | |
| 3. Rosettes on early nodes | 20 | 13.2 | 0 | 0 | ||
| 4. Combined phenotypes of 2 and 3 | 15 | 9.9 | 0 | 0 | ||
| 5. Homeotic transformation | 6 | 4.0 | 0 | 0 | ||
| 6. Flower defects and infertility | 3 | 2.0 | 0 | 0 | ||
| 7. Flowering time, days‡ | 35–70 | 35 (±3) | 35 (±2) | |||
| 8. Normal phenotypes | 68 | 45.0 | 24 | 96.0 | 83 | 100.0 |
| Total | 151 | 100.0 | 25 | 100.0 | 83 | 100.0 |
Only distinct phenotypes were shown.
With a few serrated rosette leaves.
The frequencies of flowering time were as follows: 30 plants flowered at 35–40 days, 81 at 41–50 days, 6 at 51–60 days, and 2 at 61–70 days after germination. The total number of the plants did not add up to 151 because of early senescence of some CASH plants.
In Arabidopsis, development of the first two leaves is symmetrical and is initiated during embryogenesis. In some CASH plants, the first and second pairs of leaves developed asymmetrically (Fig. 4 B and C). In two plants, the first two leaves were elongated with little expansion and developed into “needle-like” structures (Fig. 4D). Approximately 9% of the transgenic plants exhibited early developmental abnormalities, and the majority died within about 2 weeks on media. As a result, we could not examine gene expression patterns in these plants. However, the data suggest that histone deacetylation is required for coordinated gene expression during early development, including embryogenesis. The surviving seedlings developed into various phenotypes, depending on the stages of gene expression affected.
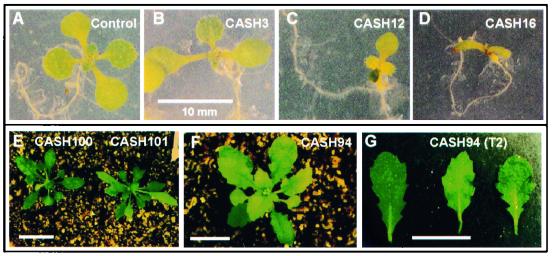
Figure 4.
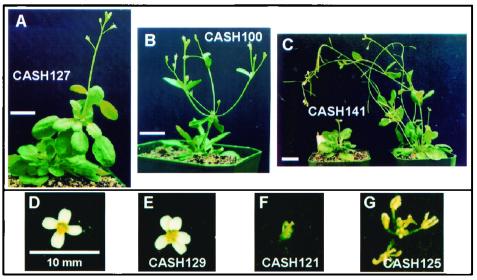
Phenotypic variation at early developmental stages in CASH plants. (A–E) Photographs of 8-day-old seedlings, showing development of asymmetric leaves in CASH3 (B) and CASH12 (C), narrow and elongated leaves in CASH16 (D), and spade-shaped leaves in CASH100 and 101 (E). The control seedling is shown in A. (F) Photograph of a serrated plant (CASH94). (G) The picture of leaves collected at rosette positions 4, 6, and 8 from the same plant after two generations of selfing. (Bars = 10 mm. The same scale is used from A to D.)
Serration of leaf margins is controlled by a single gene, SERRATE, in Arabidopsis (28). SERRATE encodes a zinc-finger protein that may be involved in transcriptional regulation. Wild-type Arabidopsis leaves are round with little or no serration in early rosette leaves. In some CASH plants (e.g., CASH94), the early leaves were heavily serrated (Fig. 4F). Moreover, SERRATE was present in the subsequent generations (Fig. 4G) in the plants homozygous for the CASH transgene. The data suggest that ectopic expression of serrate alleles is induced by blocking histone deacetylation (Fig. 3, lane 11). Alternatively, a repressor that is turned on by inhibiting histone deacetylation indirectly activates the serrate.
Bract formation is a transition from vegetative to reproductive (flower) development; cauline leaves and inflorescence are developed from bracts. Some CASH plants (e.g., CASH127) developed aerial rosette-like structures, usually in the first two nodes (Fig. 5A). The secondary aerial rosettes behaved like the primary rosettes and developed into stem and inflorescence structures in late development stages. This phenomenon resembles homeotic transformation, as initially described in Drosophila (29). CASH100 and 101 developed narrow rosette leaves (Fig. 4E), and homeotic changes occurred as time progressed. CASH100 had elongated internodes with no apical dominance, which eventually led to the development of four equally growing branches (Fig. 5B). This plant possessed severe developmental abnormalities, including male and female sterility, reduced AtHD1 expression, and ectopic expression of SUP (Fig. 3B, lane 9).
Figure 5.
Phenotypic variation at late developmental stages in CASH plants. (A) Photograph of the CASH127 plant, showing the development of aerial rosettes in the first node. (B) Photograph of the CASH100 plant, showing no apical dominance and developing four inflorescence branches at the first node. (C) Photograph of a dwarf and late flowering plant (CASH141, Left) and a normal control plant (Right). (D–G) Photographs of various flower phenotypes, including a flower with five petals (E), no petal (F), and no petal and sepal (G). A normal Arabidopsis flower is shown in D. (Bars = 10 mm. The same scale is used from D to G.)
The developmental changes observed included a delay of the phase transition from the vegetative to the reproductive stage. Under 14 h/day of illumination, the Columbia strain typically flowered at about 35 days after germination. The CASH plants displayed prolonged juvenile stages (Fig. 5C Left), with the majority flowering at 41–70 days (Table 1). Delay of the phase transition was also observed in DNA methylation mutants (30) and antisense methyltransferase plants (31, 32). Thus, like DNA methylation, histone deacetylation plays a role in orchestrating gene expression during reproductive development.
Phenotypic Changes in CASH Transgenic Plants Are Associated with AtHD1 Reduction and Histone Hyperacetylation but with DNA Methylation.
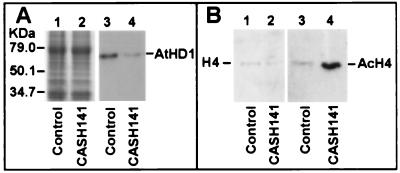
Changes in AtHD1 production and histone acetylation profiles were examined in a plant that showed a dramatic reduction in AtHD1 transcription. CASH141 had approximately 10% of AtHD1 protein produced compared with a control plant (Fig. 6A, lanes 3 and 4). As a result, the transgenic plant had an elevated level (≈10-fold) of tetraacetylated histone H4 (Fig. 6B, lanes 3 and 4). Histone H4 hyperacetylation was previously found in the yeast rpd3 null mutant (12). Thus, the AtHD1 shares a similar deacetylation function with RPD3 in yeast (11, 12) and HD1 in mammals (18). This claim is supported by the evidence that the HD1 homolog in maize functionally complements the rpd3 mutation in yeast (23).
Figure 6.
AtHD1 reduction and histone H4 hyperacetylation in CASH 141. Crude protein extracts (25 μg) or histone fractions (2 μg) were loaded onto a SDS/PAGE gel and then immunoblotted onto Immobilon-P (Millipore) or Hybond-ECL (Amersham Pharmacia). The membrane was probed with antibodies against the N terminus of AtHD1 and antibodies specific for nonacetylated (H4) or tetraacetylated histone H4 (AcH4). (A) AtHD1 in CASH141 was reduced to 10% (lane 4) of the level in a control plant (lane 3). Protein loading control is shown in an 8% SDS/PAGE stained with Coomassie blue (lanes 1 and 2). (B) Histone H4 was hyperacetylated ≈10-fold in the CASH141 (lane 4) compared with the wild-type plant (lane 3). An equal amount of nonacetylated histone H4 was detected (lanes 1 and 2).
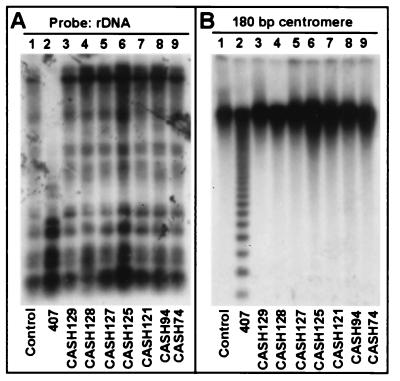
Biochemical studies suggest that DNA methylation is involved in the repression of gene transcription by recruiting HDs (3, 5). However, inhibiting HDs by trichostatin A may also induce changes in DNA methylation at some specific loci (33). To determine whether reduction in AtHD1 has any effects on changes in DNA methylation, we examined methylation status in the repetitive DNA sequences (rDNA and centromere) and a specific locus (SUP). The HapII restriction enzyme has a nucleotide-recognition site (CCGG); however; it does not digest the DNA if the inner cytosine is methylated. Among the seven transgenic plants analyzed, the methylation patterns in the rDNA (Fig. 7A, lanes 3–9) and centromere repeats (Fig. 7B, lanes 3–9) were similar to those in the control plant (Fig. 7, lanes 1). In contrast, demethylation was observed in an antisense MET1 plant (Fig. 7, lanes 2). In addition, we failed to detect any changes in DNA methylation in the SUP locus (data not shown) with the use of MobI and Sau3A, which could distinguish methylation status between wild-type and SUP alleles (34).
Figure 7.
DNA blot analysis of the genomic DNA methylation in the CASH plants and an antisense MET1 plant. Total genomic DNA (2 μg) was digested with HpaII and transferred onto Hybond-N+ membrane. (A) The blot was probed with intergenic spacer probe of the 26S rDNA (16). (B) The blot was hybridized with a 180-bp centromere repeat (30).
Discussion
Histone Deacetylation Is Involved in Epigenetic Silencing in Arabidopsis.
The role of histone deacetylation in plant gene regulation and development is unclear. Trichostatin A, a HD inhibitor, could induce transient phenotypes in Arabidopsis; the phenotypes were fewer and less severe than what was observed in the CASH plants (data not shown). However, blocking histone deacetylation by trichostatin A derepresses one parental set of rRNA genes that is normally silenced in a Brassica allotetraploid (16), indicating that histone modifications are involved in epigenetic control of gene expression in plants as in other eukaryotes. This notion has been supported by our data in the CASH plants that display developmental pleiotropy (Figs. 4 and 5). Down-regulation of AtHD1 expression changes gene expression and phenotypes at various stages of plant development from embryogenesis to flower development and seed production. Some changes are associated with epigenetic reactivation of genes that are normally silenced, for example, ectopic expression of SUP and phenotypic expression of serrated leaves, which is reminiscent of the lethal phenotypes observed in early developmental stages and male and female sterility at late stages. Many CASH plants have reduced fertility, ranging from 0 to 90% of the seed sets of wild-type plants. Seed abortion is also observed in antisense AtHD2 plants (26). AtHD1 suppression appears to have more effects than AtHD2 on plant development. Multiple HDs may be involved in various biological processes. For example, HD2 is localized in nucleoli (25), suggesting a role in rDNA chromatin organization. Sir2 protein is an NAD-dependent HD and is involved in transcriptional silencing in yeast (27).
Overexpressing antisense AtHD1 induces a wide range of phenotypes, which is likely caused by the various levels of endogenous AtHD1 suppression (Fig. 3). The levels of AtHD1 production from representative CASH plants in six groups (Table 1, phenotypes 2–7) are 90, 90, 95, 85, 40, and 10% of the control levels. Compared with the AtHD1 suppression, the levels of AtHD1 reduction correlate with the severity of phenotypes in the plants with severe abnormalities in the last two groups but not in others (Table 1 and Fig. 3B). One possibility is that the AtHD1-N antibodies crossreact with related proteins, whereas the RNA probe is more specific, so that the correlation at the RNA level is more reliable (Fig. 3). Alternatively, a defective AtHD1 (RPD3 homolog) may not be compensated for by other related proteins. Indeed, yeast RPD3 and HDA1 have slightly different effects on the specificity of deacetylating lysine residues (12).
In our previous studies, neither 5′-aza-2′-deoxycytidine nor trichostatin A induced transmissible changes in gene expression (16), which was different from results in maize, in which the phenotypes induced by 5′-aza-2′-deoxycytidine were inherited for a few generations (35). In this study, some phenotypic changes induced by antisense AtHD1 expression were similar to those induced by chemical treatments, i.e., they were not heritable. However, some phenotypes (e.g., serrate) were transmitted through meiosis (Fig. 4G) when CASH transgene was present. We postulate that some changes induced by blocking histone deacetylation either are transient or are corrected by other chromatin factors, such as DNA methylation, whereas others are heritable by maintaining specific chromatin states, as observed in mating-type and telomeric loci in yeast (36) and position-effect variegation in Drosophila (14) (see below). Indeed, we have recovered some plants that carry silenced transgenes (sensitive to kanamycin) but maintain abnormal phenotypes, implying that changes induced by epigenetic modifications on histones may be selected and maintained.
The Role of Histone Deacetylation and DNA Methylation in Plant Gene Regulation and Development.
It is evident that HDs are involved in several pathways for transcriptional repression (8, 13). The HD repressor complex, including Sin3, can be recruited to mediate gene silencing or transcriptionally competent states by sequence-specific DNA-binding proteins, such as N-CoR, SMRT, Mad/Max, and E2F-Rb (7–10). Alternatively, acetylated lysine residues can serve as signals for transcriptional silencing or activation (14). In organisms that have both DNA and histone modifications, HDs either exist within a complex containing MeCP2 or MBD2 (3, 4) or directly interact with Dnmt-1 (6), implying that DNA methylation represses gene activity through changes in histone deacetylation. Four lysine residues, 5, 8, 12, and 16 can potentially be modified. It is interesting to note that hyperacetylation of lysine 14 on H3 and lysine 5 on H4 is associated with gene activation, whereas acetylation of lysine 12 on H4 is correlated with silencing of some genes (2, 8, 14). An increase in H4 hyperacetylation induced by antisense AtHD1 expression (Fig. 6) may disrupt both negative and positive circuits of gene regulation in the CASH plants, resulting in pleiotropic effects on plant gene regulation and development.
Although CASH plants display many changes, genomic DNA methylation is maintained at the same levels as in the control plants in the rDNA, centromeres, and SUP (Fig. 7). Our data support the notion that histone deacetylation is directly involved in gene silencing, whereas DNA methylation may recruit HDs to silence genes or act upstream of histone deacetylation.
Reduction in the type and amount of HDs produced affects stochastic interactions with transcriptional factors or chromatin proteins and results in gene activation. The onset of a series of phenotypes during CASH plant development may reflect this effect on the multiple independent transgenic plants. In some plants, e.g., CASH74, aberrant phenotypes were observed during selfing, implying that inhibiting histone deacetylation might reactivate some factors, such as transposable elements, causing unstable mutations. Alternatively, if the antisense AtHD1 transgene is not silenced in subsequent generations or segregated away from a particular phenotype, it may induce additional epimutations (30). Collectively, current data suggest that epigenetic regulation involving histone modifications plays an important role in morphogenesis and possibly evolution of plant form and function.
Evidence supports that a natural variation of asymmetrical flower development in Linaria vulgaris (37) is because of epimutation in genomic DNA methylation in Cycloidea (Lcyc), a gene encoding a transcriptional factor. Also, suppression of mutations in SUP alleles in Arabidopsis is correlated with DNA methylation (34). However, MOM, a protein predicted with chromatin remodeling motifs, is associated with transcriptional gene silencing independent of DNA methylation (38). It will be interesting to know how the methylated alleles cause phenotypic changes and whether histone deacetylation is involved in the process of these epimutations.
Acknowledgments
Z.J.C. is indebted to Craig Pikaard for his advice and support during early stages of the work and Eric Richards for insightful discussions. We thank David Allis (University of Virginia, Charlottesville) for histone antibodies, Jean Finnegan (Commonwealth Scientific and Industrial Research organization, Plant Industry, Canberra, Australia) for an Arabidopsis MET1 cDNA clone, Elliot Meyerowitz (California Institute of Technology, Pasadena) for a plasmid (pHS-supL1) containing the SUP, and the Arabidopsis Stock Center for an expressed sequence tag clone (GenBank accession no. AF014824). We are grateful to Timothy Hall, Gary Hart, and anonymous reviewers for critical suggestions on improving the manuscript and to members in the Chen lab for numerous discussions. This work was supported by funds to Z.J.C. from the National Institutes of Health (NRSA GM19072) and by the Texas A&M Agricultural Experiment Station.
Abbreviations
- HD
histone deacetylase
- AtHD
Arabidopsis thaliana HD
- CASH
constitutive antisense histone deacetylase gene
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.011347998.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.011347998
References
- 1.Allfrey V G, Faulkner R, Mirsky A E. Proc Natl Acad Sci USA. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner B M. J Cell Sci. 1991;99:13–20. doi: 10.1242/jcs.99.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Nan X, Ng H H, Johnson C A, Laherty C D, Turner B M, Eisenman R N, Bird A. Nature (London) 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 4.Ng H H, Zhang Y, Hendrich B, Johnson C A, Turner B M, Erdjument-Bromage H, Tempst P, Reinberg D, Bird A. Nat Genet. 1999;23:58–61. doi: 10.1038/12659. [DOI] [PubMed] [Google Scholar]
- 5.Jones P L, Veenstra G J, Wade P A, Vermaak D, Kass S U, Landsberger N, Strouboulis J, Wolffe A P. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 6.Fuks F, Burgers W A, Brehm A, Hughes-Davies L, Kouzarides T. Nat Genet. 2000;24:88–91. doi: 10.1038/71750. [DOI] [PubMed] [Google Scholar]
- 7.Heinzel T, Lavinsky R M, Mullen T M, Soderstrom M, Laherty C D, Torchia J, Yang W M, Brard G, Ngo S D, Davie J R, et al. Nature (London) 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 8.Pazin M J, Kadonaga J T. Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 9.Brehm A, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. Nature (London) 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 10.Nicolas E, Morales V, Magnaghi-Jaulin L, Harel-Bellan A, Richard-Foy H, Trouche D. J Biol Chem. 2000;275:9797–9804. doi: 10.1074/jbc.275.13.9797. [DOI] [PubMed] [Google Scholar]
- 11.Vidal M, Gaber R F. Mol Cell Biol. 1991;11:6317–6327. doi: 10.1128/mcb.11.12.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rundlett S E, Carmen A A, Kobayashi R, Bavykin S, Turner B M, Grunstein M. Proc Natl Acad Sci USA. 1996;93:14503–14508. doi: 10.1073/pnas.93.25.14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadosh D, Struhl K. Genes Dev. 1998;12:797–805. doi: 10.1101/gad.12.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Rubertis F, Kadosh D, Henchoz S, Pauli D, Reuter G, Struhl K, Spierer P. Nature (London) 1996;384:589–591. doi: 10.1038/384589a0. [DOI] [PubMed] [Google Scholar]
- 15.Bartl S, Taplick J, Lagger G, Khier H, Kuchler K, Seiser C. Mol Cell Biol. 1997;17:5033–5043. doi: 10.1128/mcb.17.9.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z J, Pikaard C S. Genes Dev. 1997;11:2124–2136. doi: 10.1101/gad.11.16.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai H, Medrano L J, Meyerowitz E M. Nature (London) 1995;378:199–203. doi: 10.1038/378199a0. [DOI] [PubMed] [Google Scholar]
- 18.Taunton J, Hassig C A, Schreiber S L. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 19.Klee H J, Hayford M B, Kretzmer K A, Barry G F, Kishore G M. Plant Cell. 1991;3:1187–1193. doi: 10.1105/tpc.3.11.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bechtold N, Pelletier G. Methods Mol Biol. 1998;82:259–266. doi: 10.1385/0-89603-391-0:259. [DOI] [PubMed] [Google Scholar]
- 21.Finnegan E J, Dennis E S. Nucleic Acids Res. 1993;21:2383–2388. doi: 10.1093/nar/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Church G M, Gilbert W. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi V, Hartings H, Motto M. Mol Gen Genet. 1998;258:288–296. doi: 10.1007/s004380050733. [DOI] [PubMed] [Google Scholar]
- 24.Mayer K E A. Nature (London) 1999;402:769–777. doi: 10.1038/47134. [DOI] [PubMed] [Google Scholar]
- 25.Lusser A, Brosch G, Loidl A, Haas H, Loidl P. Science. 1997;277:88–91. doi: 10.1126/science.277.5322.88. [DOI] [PubMed] [Google Scholar]
- 26.Wu K, Tian L, Malik K, Brown D, Miki B. Plant J. 2000;22:19–27. doi: 10.1046/j.1365-313x.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- 27.Imai S, Armstrong C M, Kaeberlein M, Guarente L. Nature (London) 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 28.Clarke J H, Tack D, Findlay K, Van Montagu M, Van Lijsebettens M. Plant J. 1999;20:493–501. doi: 10.1046/j.1365-313x.1999.00623.x. [DOI] [PubMed] [Google Scholar]
- 29.Morata G, Kerridge S. Nature (London) 1981;290:778–781. doi: 10.1038/290778a0. [DOI] [PubMed] [Google Scholar]
- 30.Kakutani T, Jeddeloh J A, Flowers S K, Munakata K, Richards E J. Proc Natl Acad Sci USA. 1996;93:12406–12411. doi: 10.1073/pnas.93.22.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronemus M J, Galbiati M, Ticknor C, Chen J, Dellaporta S L. Science. 1996;273:654–657. doi: 10.1126/science.273.5275.654. [DOI] [PubMed] [Google Scholar]
- 32.Finnegan E J, Peacock W J, Dennis E S. Proc Natl Acad Sci USA. 1996;93:8449–8454. doi: 10.1073/pnas.93.16.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selker E U. Proc Natl Acad Sci USA. 1998;95:9430–9435. doi: 10.1073/pnas.95.16.9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobsen S E, Meyerowitz E M. Science. 1997;277:1100–1103. doi: 10.1126/science.277.5329.1100. [DOI] [PubMed] [Google Scholar]
- 35.Ronchi A, Petroni K, Tonelli C. EMBO J. 1995;14:5318–5328. doi: 10.1002/j.1460-2075.1995.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braunstein M, Rose A B, Holmes S G, Allis C D, Broach J R. Genes Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- 37.Cubas P, Vincent C, Coen E. Nature (London) 1999;401:157–161. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- 38.Amedeo P, Habu Y, Afsar K, Scheid O M, Paszkowski J. Nature (London) 2000;405:203–206. doi: 10.1038/35012108. [DOI] [PubMed] [Google Scholar]