Abstract
The signalosome (CSN) is a conserved multiprotein complex involved in regulation of eukaryotic development and is also required to activate ribonucleotide reductase for DNA synthesis. In Aspergillus nidulans, csnD/csnE are key regulators of sexual development. Here, we investigated whether the csnD/csnE genes are involved in the DNA damage response in this fungus. The growth of the csnD/csnE deletion mutants was reduced by subinhibitory concentrations of hydroxyurea, camptothecin, 4-nitroquinoline oxide, and methyl methanesulfonate. A. nidulans increases csnD/csnE mRNA levels when it is challenged by different DNA-damaging agents. There is no significant transcriptional induction of the csnE promoter fused with lacZ gene in the presence of DNA-damaging agents, suggesting that increased mRNA accumulation is due to increased mRNA stability. Septation was not inhibited in the csnD/csnE deletion mutants while ΔuvsB ΔcsnE presented an increase in septation upon DNA damage caused by methyl methanesulfonate, suggesting that uvsBATR and csnE genetically interact during checkpoint-dependent inhibition of septum formation. The double ΔcsnD/ΔcsnE ΔnpkA mutants were more sensitive to DNA-damaging agents than were the respective single mutants. Our results suggest that csnD/csnE genes are involved in the DNA damage response and that NpkA and UvsBATR genetically interact with the signalosome.
THE constitutive photomorphogenesis complex 9 (COP9), termed the COP9 signalosome (CSN), is a conserved nuclear-enriched multiprotein complex composed of eight subunits that are involved in regulation of eukaryotic development and the activation of ribonucleotide reductase for DNA synthesis (Schwechheimer and Deng 2001; Bech-Otschir et al. 2002; Cope and Deshaies 2003; Nielsen 2003; Schwechheimer 2004). It has been found in plants, mammals, Drosophila melanogaster, Schizosaccharomyces pombe, and Aspergillus nidulans (Schwechheimer 2004). The COP9 signalosome seemed to be absent from Saccharomyces cerevisiae; however, the verification of results from large-scale genomics and proteomics studies has recently resulted in the isolation of a CSN-related complex from this organism (Maytal-Kivity et al. 2002; Wee et al. 2002; Schwechheimer 2004). The COP9 signalosome was initially identified from Arabidopsis thaliana mutants with a light-brown seedling phenotype when grown in the dark (Chamovitz et al. 1996; Wei and Deng 1999). Later, most of the COP loci were subsequently found to be required for COP1 to go into the nucleus (Schwechheimer 2004). It was shown that COP1 negatively controls the levels of Hy5 and HyH, transcriptional regulators involved in photomorphogenesis, through subcellular localization and proteolysis (Osterlund et al. 2000).
The specific degradation of proteins in eukaryotes is mediated by the 26S proteasome, which is divided into the proteolytic core particle (CP) and two presumably identical 19S regulatory particles (RP) that are placed at either end of the 20S CP (Baumeister et al. 1998). The 19S RP consists of a base and a lid, and the eight subunits that form the lid are paralogs of the eight CSN subunits (Schwechheimer and Deng 2001). It has been suggested that the CSN and 19S RP lid have a common ancestor and possess similar biochemical properties (Schwechheimer and Deng 2001). They are characterized through the presence of two signature domains known as the proteasome, COP9 signalosome, initiation factor 3/proteasome subunits, Int-6, Nip-1, and TRIP-15 (PCI/PINT) and the Mrp1p, Pad1 N-terminal family (MPN/MVO34) protein domains (Glickman et al. 1998; Kim et al. 2001). Protein degradation by the 26S proteasome is usually headed by protein ubiquitylation, a process mediated by an E1 ubiquitin-activating enzyme, an E2 ubiquitin-conjugating enzyme, and an E3 ubiquitin ligase (for a review, see Fang and Weissman 2004). E3 ubiquitin ligases interact directly with protein substrates to mediate their ubiquitylation together with associated E2 ubiquitin-conjugating enzymes. It is noteworthy that most of the proteins that interact with the signalosome either are directly involved in protein degradation or are known to be regulated by protein degradation, such as transcription factors and cell cycle regulators (Schwechheimer and Deng 2001). The CSN directly interacts with E3 ubiquitin ligases (Scwechheimer 2004) and it is suggested that it regulates their activity toward its protein substrates by deneddylation of the E3 cullin subunit (Lyapina et al. 2001; Zhou et al. 2001; Cope et al. 2002; Yang et al. 2002) and by phosphorylation of the target proteins (Bech-Otschir et al. 2001; Sun et al. 2002).
In mammalian cells, CSN is involved in several processes such as the control of hormone signaling and tumor growth by regulation of c-Jun and p53 protein levels (Li et al. 2000; Pollmann et al. 2001) and inhibition of the mouse cyclin-dependent kinase inhibitor p27KIP1 degradation and blockage of the G1-S phase progression via deneddylation of SCF Cul1 (Tomoda et al. 1999; Yang et al. 2002). In insects and plants, the COP9 signalosome is an essential regulator of development and its malfunction results in postembryonic lethality (Wei et al. 1994; Freilich et al. 1999). Deletion mutants in S. pombe csn1 and csn2 mutants are delayed in S phase and are sensitive to UV light and ionizing radiation (Mundt et al. 2002). The S. pombe Csn1 and Csn2 subunits revealed their role in positively regulating the activity of the ribonucleotide reductase (RNR) through a proteolysis of the replication inhibitor Spd1 (Liu et al. 2003).
A. nidulans has been used as a model genetic system for the study of cell cycle control and DNA damage response (for reviews, see Kafer and May 1998; Aist and Morris 1999; Bruschi et al. 2001; Goldman et al. 2002; Fagundes et al. 2003, 2004; Semighini et al. 2003; for a review, see Goldman and Kafer 2004; Osmani and Mirabito 2004). A. nidulans forms asexual spores (conidia), which contain a single nucleus arrested in G1 (Bergen and Morris 1983). During spore germination, conidia undergo an initial period of isotropic expansion before switching to polarized growth and forming an elongating germ tube. Two protein kinases, the NimXcdc2 and NimA, are coordinately required to initiate mitosis in A. nidulans (Osmani and Ye 1996; Ye et al. 1997). Like other eukaryotic cells, the DNA damage checkpoint functions via Y15 phosphorylation of the NimXcdc2 in A. nidulans (Ye et al. 1997). Loss of such checkpoint control regulation over mitosis can also cause defects in DNA rereplication after mitosis (De Souza et al. 1999). Although the Wee1 ortholog AnkA and the Cdc25 ortholog NimT control Y15 phosphorylation of NimX (Osmani et al. 1991; Kraus and Harris 2001), it is not clear how their activity and/or localization are influenced by DNA damage. De Souza et al. (1999) have shown that both A. nidulans uvsBATR and uvsDATRIP are involved in the G2/M checkpoint in response to DNA damage. Ye et al. (1997) provided evidence that at least two S-phase checkpoint mechanisms control mitosis in A. nidulans. The first responds to the rate of DNA replication and inhibits mitosis via tyrosine phosphorylation of NimXcdc2. If DNA replication is arrested, lack of tyrosine-phosphorylated NimXcdc2 also cannot promote mitosis because of the presence of a second checkpoint mechanism over mitotic initiation, which involves the function of BimEAPC (the homolog of the anaphase-promoting complex, APC). The DNA damage checkpoint also regulates septation in A. nidulans by modulating the activity of NimXcdc2 and requiring functional AnkA (Harris and Kraus 1998; De Souza et al. 1999). Septum formation is triggered by high levels of NimXcdc2 activity and is inhibited by DNA damage in a checkpoint-dependent manner (Harris and Kraus 1998). Harris and Kraus (1998) showed that mutations in uvsBATR abolish the cell cycle delay and inhibition of septum formation triggered by the exposure of predivisional hyphae to DNA-damaging agents. Semighini et al. (2003) suggested that regulation of septation in A. nidulans is dependent not only on the uvsBATR gene but also on the Mre11 complex.
Recently, Busch et al. (2003) identified two components of the COP9 signalosome, csnD/csnE, as novel regulators of sexual development in A. nidulans. The deletion of these two genes resulted in viable strains with mutant phenotypes, the most critical a block in maturation of cleistothecial primordia. Deneddylation of cullins is mediated by the fifth subunit of the signalosome and the characteristic JAB1/MPN/Mov34 metalloenzyme (JAMM) motif, which is required for this metalloprotease activity, is present in A. nidulans CsnE, suggesting that this gene encodes the deneddylase activity of the fungal signalosome (Cope et al. 2002). Here, we investigated whether the csnD/csnE genes are involved in the DNA damage response in this fungus. We observed not only that these deletion mutant strains impaired growth in the presence of DNA-damaging agents, but also that septation is not inhibited in the csnD/csnE deletion mutants upon DNA damage caused by methyl methanesulfonate (MMS). Furthermore, we have also seen genetic interactions among csnD/csnE genes, the cdc2-related kinase NpkA, and UvsBATR during A. nidulans DNA damage response.
MATERIALS AND METHODS
Strains, media, and methods of UV treatment:
A. nidulans strains used are described in Table 1. Media were of two basic types: (1) a simple yeast extract complete medium with the three variants YAG (2% glucose, 0.5% yeast extract, 2% agar, trace elements), YUU (YAG supplemented with 1.2 g/liter each of uracil and uridine), and liquid YG medium (YAG but without 2% agar) and (2) a modified minimal medium (MM) of 1% glucose, original high nitrate salts, trace elements, 2% agar, pH 6.5 or a minimal medium without glucose (MC). Trace elements, vitamins, and nitrate salts are described by Kafer (1977, Appendix). Standard genetic techniques for A. nidulans were used for all strain constructions (Kafer 1977).
TABLE 1.
A. nidulans strains
| Strains | Genotypes | References |
|---|---|---|
| GR5 | pyrG89; wA3; pyroA4 | FGSC A773 |
| R21 | pabaA1 yA2 | FGSC A234 |
| AGB152 | pyroA4; pyrG89 | Busch et al. (2003) |
| AGB195 | pyroA4; pyrG89; ΔcsnD∷pyr4 | Busch et al. (2003) |
| AGB209 | pyroA4; pyrG89; ΔcsnE∷pyr4 | Busch et al. (2003) |
| AAH14 | pabaA1 yA2; ΔuvsB; argB2 | Hofmann and Harris (2000) |
| MV3 | argB2; pyrG89; yA2; ΔnpkA∷pyrG | Kress-Fagundeset al. (2004) |
| JL195-3 | ΔcsnD∷pyr4; ΔnpkA∷pyrG | This work |
| JL195-14 | ΔcsnD∷pyr4; ΔuvsB∷argB | This work |
| JL209-3 | ΔcsnE∷pyrG; ΔnpkA∷pyrG | This work |
| JL209-14 | ΔcsnE∷pyrG; ΔuvsB∷argB | This work |
| APK35 | ankA; pabaA1 | Kraus and Harris (2001) |
| WG 355 | biA1, bgaO, argB2 | Van Gorcom et al. (1986) |
| AGB 246 | biA1; bgaO; argB2, lacZ <argB> | This work |
| AGB 248 | biA1; bgaO; argB2, 5′csnE∷lacZ <argB> | This work |
| FGSC A4 | Glasgow wild type | FGSC A4 |
| A776 | PabaA1; acrA1; bimE7; riboB2; chaA1 | FGSC A776 |
For the UV-light viability assays, conidiospores (dormant in a quiescent G0 state) were suspended in 0.2% Tween-20 and plated out on YUU plates (∼100 conidia/plate). The plates were then irradiated immediately with UV using a UV Stratalinker 1800 (Stratagene, La Jolla, CA) and incubated at 37° for 48 hr to determine UV sensitivity of nondividing cells. To determine UV survival of dividing cells, conidiospores on YUU plates were first allowed to germinate for 4.5 hr in a 37° incubator for colony formation. By this time the germinated spores had entered the cell cycle and were about to undergo the first mitosis. These germlings were UV irradiated on the plates and then similarly incubated at 37° for 48 hr. Viability was determined as the percentage of colonies on treated plates compared to untreated controls.
Construction of the 5′csnE∷lacZ fusion plasmid:
Primers ES59 (5′-TGA GGATCC GGC TTT CTC GTC AAC CAG-3′) and ES60 (5′-TAG GGA TCC CAT GAT GAT TGT CAG GTG-3′) containing BamHI restriction sites were used for amplifying 1.0 kb of the 5′ regulatory region of the A. nidulans csnE gene from genomic DNA from the AGB152 strain. The 1.0-kb PCR fragment was fused to lacZ in the unique BamHI site of plasmid pAN923-41B (Van Gorcom et al. 1986), resulting in pME 2817. The plasmid-borne argB wild-type gene was mutated by filling in the unique BglII site within the argB gene with PolIK, creating an insertion mutant argB allele (Punt et al. 1990). This mutation allows the selection of arginine prototrophy transformants generated by recovering the wild-type allele by crossing over with the argB2 mutation of WG355 strain.
A. nidulans transformation was performed as described (Eckert et al. 2000). The strain WG355 was transformed with plasmids pME2817 or pAN923-41B. Arginine prototrophy transformants were tested for single-copy integrations at the chromosomal argB gene locus by Southern blot analysis (Southern 1975) and by using the gene images random prime labeling and detection system (Amersham, Freiburg, Germany). The strain AGB 246 containing pAN923-41B serves as negative control. The AGB 248 strain contains the 5′csnE∷lacZ fusion as single copy.
Specific β-galactosidase activity assay:
A. nidulans strains were grown and harvested under the same conditions as for RNA isolation. Mycelia were harvested by filtration through no. 1 Whatman filter, washed thoroughly with sterile water, and quickly frozen in liquid nitrogen. About 300 μl of this ground mycelia was mixed with 500 μl B+-buffer (100 mm Tris-HCl, pH 7.5, 200 mm NaCl, 20% glycerol, 5 mm EDTA, pH 8.0, and freshly added 1 μl/ml β-ME) and 5 μl/ml 200× 100 mm p-aminobenzamidin-HCL, 100 mm Na-p-tosyl-l-lysin-chlormethylketon, 100 mm Na-p-tosyl-l-phenylalanin-chlormethylketon, and 100 mm o-phenanthrolin and 100 mm phenylmethylsulfonyl fluoride (PIM) and vortexed four times for 15 sec. After centrifugation at 4° for 10 min, the supernatant was used directly for further analysis. Protein concentrations were determined according to Bradford (1976). β-Galactosidase assays were carried out at 28° as described by Miller (1972) using 0.5–5.0 μl of extracts.
Methods of replication checkpoint response:
For the mitosis assay, conidiospores were inoculated onto coverslips in YUU medium with 0, 6, or 100 mm of hydroxyurea (HU). After 5–7 hr incubation at 30°, coverslips with adherent germlings were transferred to fixative solution (3.7% formaldehyde, 50 mm sodium phosphate buffer, pH 7.0, 0.2% Triton X-100) for 30 min at room temperature. Then they were briefly rinsed with PBS buffer (140 mm NaCl, 2 mm KCl, 10 mm NaHPO4, 1.8 mm KH2PO4, pH 7.4) and incubated for 5 min in a solution with 100 ng/ml of 4′,6-diamidino-2-phenylindole (DAPI) (Sigma Chemical) and 100 ng/ml of calcofluor (fluorescent brightener, Sigma Chemical). After incubation with the dyes, they were washed with PBS buffer for 5 min at room temperature and then rinsed in distilled water and mounted on the slides. The material was photographed using a Zeiss epifluorescence microscope. The number of nuclei was assessed by DAPI staining. Germlings that had two or more nuclei after the HU incubation were scored as having a nonfunctional checkpoint response.
For the viability assay, 1.0 × 108 conidia were inoculated in YUU medium with 0, 6, or 100 mm of HU and incubated in a reciprocal shaker (250 rpm) at 30° for 7 hr. The conidiospores were washed with water, conveniently diluted and plated on YUU, and incubated at 30° for 48 hr. Viability was determined as the percentage of grown colonies on plates with drug-treated conidiospores compared to untreated controls.
RNA isolation:
A total of 1.0 × 107 conidia/ml were used to inoculate 50 ml of liquid cultures that were incubated in a reciprocal shaker at 37° for 16 hr. Mycelia were aseptically transferred to fresh YG medium in the presence or absence of drugs for 30, 60, 90, 120, or 240 min. The following concentrations of chemicals were used: 25 μm of camptothecin (CPT), 0.5 μg/ml of 4-nitroquinoline oxide (4-NQO), 0.003% of MMS, and 0.6 μg/ml of bleomycin (BLEO). Mycelia were harvested by filtration through no. 1 Whatman filter, washed thoroughly with sterile water, quickly frozen in liquid nitrogen, and disrupted by grinding, and total RNA was extracted with Trizol (Life Technologies). Ten micrograms of RNA from each treatment were then fractionated in 2.2 m formaldehyde and 1.2% agarose gel, stained with ethidium bromide, and then visualized with UV light. The presence of intact 28S and 18S ribosomal RNA bands was used as a criterion to assess the integrity of the RNA. RNAse-free DNAse treatment was done as previously described by Semighini et al. (2002).
Real-time PCR reactions:
All the PCR and RT-PCR reactions were performed using an ABI Prism 7700 sequence detection system (Perkin-Elmer Applied Biosystems). Taq-Man EZ RT-PCR kits (Applied Biosystems, Foster City, CA) were used for RT-PCR reactions. The thermal cycling conditions composed an initial step at 50° for 2 min, followed by 30 min at 60° for reverse transcription, 95° for 5 min, and 40 cycles at 94° for 20 sec and 60° for 1 min. Taq-Man Universal PCR master mix kit was used for PCR reactions. The thermal cycling conditions composed an initial step at 50° for 2 min, followed by 10 min at 95°, and 40 cycles at 95° for 15 sec and 60° for 1 min. The reactions and calculations were performed according to Semighini et al. (2002). Table 2 describes the primers and Lux fluorescent probes (Invitrogen, San Diego) used in this work.
TABLE 2.
Primers and fluorescent probes used in this work
| Primers and probes | Sequences | Genesa |
|---|---|---|
| csnD_559RL | 5′-CACTTCTTGGGAAAGCCGGAAG[FAM]G-3′ | csnD AF236662 |
| csnD_559RL_545FU | 5′-CCGAGCAAGATCGAAGACCA-3′ | csnD AF236662 |
| csnE_342FL | 5′-GACCATGACGAAGCAAACGAGTATATGG[FAM]C-3 | csnE EAA64961 |
| csnE_342FL_402RU | 5′-CCCGTAGCCAGGGTGACTG-3′ | csnE EAA64961 |
| rib_reduct_820RL | 5′-CACACCAGGCAGGCAAAGTCGGTG[FAM]G-3′ | rnrA AAG40862 |
| rib_reduct_820RL_768FU | 5′-GGCTGAAGAAGCGAGGCTTG-3′ | rnrA AAG40862 |
| rns_2P_reduct_1560FL | 5′-GACACCGCCCGATTGCTCTTGGTG[FAM]C-3′ | rnsA XM_408517.1 |
| rns_2P_reduct_1560RU | 5′-GGCTTCAGCCGAATCGAAAG-3′ | rnsA XM_408517.1 |
| tubC_525FL | 5′-CACTTTATGCCGTCGCCGAAAG[FAM]G-3′ | tubC M17520 |
| tubC_525FL_583RU | 5′-GCAGAATGTCTCGTCCGAATG-3′ | tubC M17520 |
| uvsC_429FL | 5′-GACGGTTGCCATACCCTTGCCG[FAM]C-3′ | uvsC Z80341 |
| uvsC_429FL_450RU | 5′-CTTCGCCGCACCCAT-3′ | uvsC Z80341 |
FAM, 6-carboxyfluorescein.
NCBI access numbers follow gene name.
RESULTS
The csnD/csnE genes are involved in different aspects of the DNA damage response in A. nidulans:
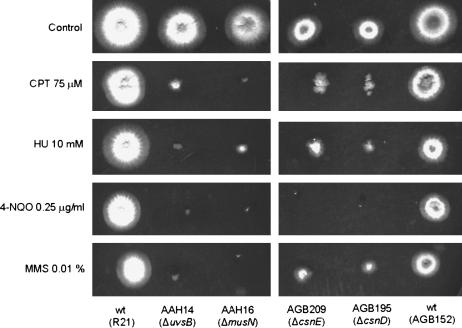
As a preliminary step to assess the involvement of the csnD/csnE genes in the DNA damage response, we verified their sensitivity to DNA-damaging agents. As can be seen in Figure 1, the ΔcsnD/ΔcsnE mutant strains are more sensitive to CPT, HU, 4-NQO, and MMS. We have also observed that germinating conidia of the csnD/csnE inactivation strains have increased sensitivity to UV light while there are no significant differences between the csnD/csnE inactivation and the wild-type strains in terms of sensitivity of quiescent conidia to UV light (data not shown).
Figure 1.
Growth phenotypes of the ΔcsnD and ΔcsnE mutants. Strains R21 and AGB152 (wild type), AAH14 (ΔuvsBATR), AAH16 (ΔmusNRecQ), AGB195 (ΔcsnD), and AGB209 (ΔcsnE) were grown for 72 hr at 37° in YUU medium and YUU 75 μm CPT, YUU 10 mm HU, YUU 0.25 μg/ml 4-NQO, and YUU 0.01% MMS.
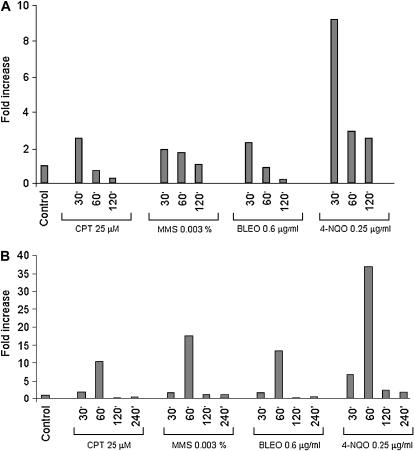
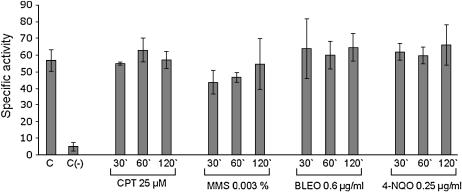
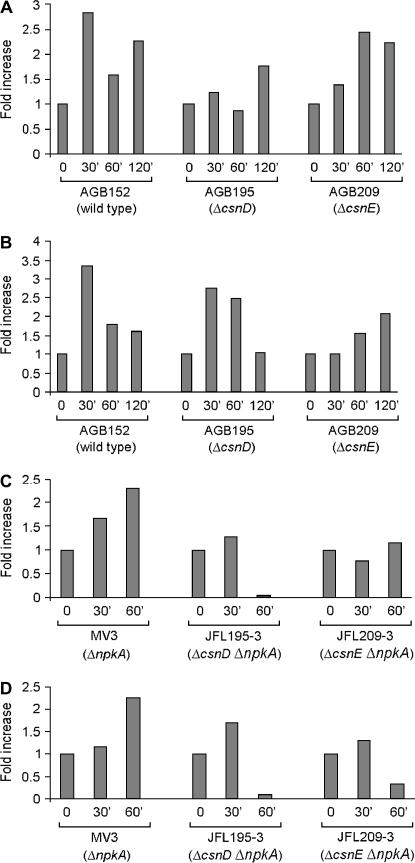
We examined the mRNA expression of the csnD/csnE in the presence of DNA-damaging agents using real-time RT-PCR. A. nidulans wild type was grown in the absence of any drug and transferred to a specific concentration of a DNA-damaging agent for 30, 60, 120, and 240 min, and then RNA was isolated and analyzed for the expression of these genes (Figure 2). The csnD gene expression was increased after 30 min growth in the presence of MMS (∼2 times), CPT and BLEO (∼3 times), and 4-NQO (∼9 times), and after 60 and 120 min growth in the presence of 4-NQO (∼3 times). The csnE gene displays a comparable profile but with much higher expression than that of csnD: mRNA levels were increased after 60 min growth in the presence of CPT (∼10 times), MMS (∼17 times), and BLEO (∼13 times), and after 30 and 60 min in the presence of 4-NQO (∼7 and 37 times, respectively). These results indicate that A. nidulans has increased csnD/csnE mRNA levels when it is challenged by different DNA-damaging agents. To verify whether these increased mRNA levels were due to transcriptional induction or to increased mRNA stability, we fused the csnE promoter with the lacZ gene and evaluated its in vivo expression in the presence of DNA-damaging agents. As it can be seen in Figure 3, there is no significant induction at the promoter level in the presence of DNA-damaging agents. These results suggested that the observed increased csnE mRNA accumulation in the presence of DNA-damaging agents is due to increased mRNA stability.
Figure 2.
Fold increase in csnD/csnE RNA levels in response to DNA-damaging agents. Mycelia were grown in the absence of any drug, 25 μm CPT, 0.003% MMS, 0.6 μg/ml BLEO, or 0.25 μg/ml 4-NQO for 30, 60, 120, and 240 min. Real-time RT-PCR was the method used to quantify the mRNA. The measured quantity of the csnD/csnE mRNA in each of the treated samples was normalized using the CT values obtained for the tubC RNA amplifications run in the same plate. The relative quantitation of csnD/csnE and tubulin gene expression was determined by a standard curve (i.e., CT values plotted against the logarithm of the DNA copy number). Results of four sets of experiments were combined for each determination; means are shown. The values represent the number of times that the genes are expressed compared to the wild-type control grown without any drug (represented absolutely as 1.00).
Figure 3.
Specific β-galactosidase activity of the A. nidulans 5′csnE∷lacZ strain (AGB248) 30, 60, and 120 min after addition of DNA-damaging agents as indicated. Each value represents the mean of two independent measurements with standard deviations not exceeding 20%. The control shows the mean specific β-galactosidase activity of strain AGB248 grown without drug. The negative [C(−)] control strain AGB246 containing lacZ without any 5′ region did not show significant β-galactosidase activity. The applied drugs are CPT, MMS, BLEO, and 4-NQO.
In A. nidulans, septation is inhibited by DNA damage in a checkpoint-dependent manner (Harris and Kraus 1998). We determined whether septation could be inhibited in ΔcsnD/ΔcsnE mutants by DNA damage caused by subinhibitory concentrations of MMS. Both mutants had normal septation levels in the absence of DNA damage when compared to the wild type (Table 3). The septation levels were dramatically reduced in wild-type mutant strain in the presence of DNA damage (Table 3). However, the septation levels were increased when the ΔcsnD/ΔcsnE mutants were grown in the presence of MMS (30.0 and 36.7%, respectively, against 7.5% from the wild-type strain; Table 3). These results suggest that the csnD/csnE signalosome genes are involved in the checkpoint-dependent inhibition of septum formation in A. nidulans.
TABLE 3.
Percentage of septation in A. nidulans germlings
| Strainsa | Control | + MMSb |
|---|---|---|
| AGB152 (wild type) | 100.0 ± 0.0 | 7.5 ± 2.1 |
| AGB195 (ΔcsnD) | 98.0 ± 3.5 | 30.0 ± 5.0 |
| AGB209 (ΔcsnE) | 96.7 ± 3.1 | 36.7 ± 6.7 |
| AAH14 (ΔuvsB) | 93.5 ± 6.4 | 22.5 ± 0.7 |
| MV3 (ΔnpkA) | 100.0 ± 0.0 | 23.5 ± 0.7 |
| JFL195-3(ΔnpkA ΔcsnD) | 100.0 ± 0.0 | 11.7 ± 4.0 |
| JFL195-14 (ΔuvsB ΔcsnD) | 100.0 ± 0.0 | 11.7 ± 6.2 |
| JFL209-3 (ΔnpkA ΔcsnE) | 100.0 ± 0.0 | 18.3 ± 7.0 |
| JFL209-14 (ΔuvsB ΔcsnE) | 100.0 ± 0.0 | 60.0 ± 0.0 |
All the experiments are the average of three independent experiments with 100 germlings evaluated in each of them.
Conidia were germinated at 37° for 16 hr in YG medium in the absence or presence of 0.0015% MMS. Since these strains are MMS sensitive, a higher concentration of MMS was used for the wild type (0.005%). Septation was evaluated by calcofluor staining.
One A. nidulans gene that has been shown to be induced in the presence of DNA damage is uvsCRAD51 (van Heemst et al. 1997). The UvsCRAD51 is important for the initial steps of the homologous recombination by binding the free ends of the DNA double strands (for a review, see Nyberg et al. 2002). In yeast cells, Rad51 homologs form irradiation-induced subnuclear foci (Gasior et al. 2001; Caspari et al. 2002). We examined the transcription of the uvsCRAD51 in the ΔcsnD/ΔcsnE mutants exposed to CPT. Thus, the mutants and the corresponding wild-type strain were grown in the absence of CPT and then transferred to complete medium containing 25 μm CPT for 30 to 120 min. After 30 min exposure to CPT, there is ∼4.5-fold induction of the uvsCRAD51 gene in the wild-type and the mutant strains (Table 4). After 60 min exposure to CPT, the expression was still comparable among the strains and it had a variation of 6.84- to 10.9-fold induction; however, after 60 min induction, the expression of the uvsCRAD51 has decreased to 2.42- and 8.8-fold induction in the ΔcsnD/ΔcsnE mutants when compared to 14.46-fold induction in the wild-type strain. These results suggest that there is decreased uvsCRAD51 gene expression in the ΔcsnD/ΔcsnE mutants when they are exposed to an agent that causes double-strand breaks (DSBs), such as CPT. Moreover, since the activation of uvsCRAD51 mRNA expression is not affected but decreased, it is likely that the csnD/csnE inactivation mutants have a defect in sustaining the DNA damage response rather than a defect in activating it.
TABLE 4.
Expression of the uvsCRAD51 gene in the A. nidulans wild-type and csnD/csnE inactivation mutants
| Strainsa | 30 min | 60 min | 120 min |
|---|---|---|---|
| AGB152 (wild type) | 4.60 ± 0.98 | 7.65 ± 0.17 | 14.46 ± 2.72 |
| AGB195 (ΔcsnD) | 4.50 ± 0.86 | 6.84 ± 0.79 | 2.42 ± 0.06b |
| AGB209 (ΔcsnE) | 4.50 ± 0.27 | 10.9 ± 1.60 | 8.80 ± 0.40b |
The strains were grown 16 hr in YG medium and then mycelia was transferred to a fresh YG medium plus 25 μm CPT. The results (mean ± standard deviation) are expressed as the number of times the uvsCRAD51 gene is more expressed than the control without CPT. Statistical differences were determined by ANOVA followed, when significant, by the Newman-Keuls Multiple Comparison Test, using Sigma Stat statistical software (Jandel Scientifics). P < 0.05 was considered statistically significant.
Significantly different from the wild type (P < 0.002).
HU is an inhibitor of ribonucleoside diphosphate reductase, the rate-limiting step enzyme in deoxyribonucleotide (dNTP) biosynthesis. Depletion of dNTPs activates the DNA replication checkpoint, which slows progression through S phase (Desany et al. 1998). Furthermore, initiation of DNA replication in the presence of high levels of HU causes DNA DSBs (Merrill and Holm 1999). Since HU is an effective inhibitor of DNA synthesis in A. nidulans (Bergen and Morris 1983), we verified whether the csnD/csnE genes could play a role in the S-phase checkpoints by examining whether the ΔcsnD/ΔcsnE mutant strains could survive a transient period of growth in the presence of HU. Two different assays were used to verify if DNA replication checkpoint response is impaired in mutant strains (Fagundes et al. 2004). The first assay (i.e., the mitosis assay) monitors mitosis in mutant and wild-type strains incubated in 6 or 100 mm of HU for 5–7 hr. The number of nuclei was assessed by DAPI staining, and a defect in the mitosis assay is defined as an increase in the number of germlings with two or more nuclei after the HU incubation (Table 5). The second assay (i.e., the viability assay) assesses germling viability after incubation for 6 hr in the presence or absence of 6 or 100 mm of HU (Table 6). Both assays measure the state of the replication checkpoint response. The ΔcsnE strain showed defects in the mitosis assay (6 mm) (Table 5). Taken together, all these evidences strongly indicate that csnD/csnE signalosome genes are involved in the A. nidulans DNA damage response.
TABLE 5.
Mitosis assay from A. nidulans wild-type and mutant strains
| HUa | 0 mm | 6 mm | 100 mm |
|---|---|---|---|
| AGB152 (wild type) | 61.3 ± 2.5 | 5.3 ± 3.2 | 0.0 ± 0.0 |
| AGB195 (ΔcsnD) | 58.5 ± 5.5 | 8.3 ± 4.6 | 2.5 ± 1.3 |
| AGB209 (ΔcsnE) | 62.0 ± 5.1 | 12.4 ± 1.7b | 0.6 ± 0.5 |
| MV3 (ΔnpkA) | 67.0 ± 3.0 | 27.3 ± 3.2b | 2.7 ± 1.2 |
| AAH14 (ΔuvsB) | 61.5 ± 7.8 | 16.5 ± 2.1b | 15.5 ± 0.7b |
| JFL195-3 (ΔcsnD ΔnpkA) | 68.5 ± 1.3 | 11.3 ± 0.9 | 3.0 ± 1.4 |
| JFL195-14 (ΔcsnD ΔuvsB) | 55.8 ± 9.8 | 5.5 ± 1.0 | 0.5 ± 0.5 |
| JFL209-3 (ΔcsnE ΔnpkA) | 51.2 ± 6.4 | 3.5 ± 0.6 | 0.0 ± 0.0 |
| JFL209-14 (ΔcsnE ΔuvsB) | 55.7 ± 3.1 | 4.3 ± 0.9 | 0.0 ± 0.0 |
Percentage of germlings that had two or more nuclei after the HU incubation were scored as germlings that did not have mitosis arrest. All the results are the average of determinations from three independent experiments with 100 germlings evaluated in each. Germlings that did not have mitosis arrest were scored. The results were expressed as mean ± standard deviation. Statistical differences were determined by ANOVA followed, when significant, by the Newman-Keuls Multiple Comparison Test, using Sigma Stat statistical software (Jandel Scientifics). P < 0.05 was considered statistically significant.
Significantly different from the wild type (P < 0.004).
TABLE 6.
Viability assay from A. nidulans wild-type and mutant strains
| HUa | 6 mm | 100 mm |
|---|---|---|
| AGB152 (wild type) | 63.0 ± 9.0 | 64.0 ± 10.0 |
| AGB195 (ΔcsnD) | 48.0 ± 7.0b | 46.0 ± 9.0b |
| AGB209 (ΔcsnE) | 53.0 ± 7.0 | 51.0 ± 8.0 |
| MV3 (ΔnpkA) | 87.0 ± 7.0 | 98.0 ± 3.0 |
| AAH14 (ΔuvsB) | 75.0 ± 13.0 | 78.5 ± 11.7 |
| JFL195-3 (ΔcsnD ΔnpkA) | 56.0 ± 5.0 | 53.0 ± 5.0 |
| JFL195-14 (ΔcsnD ΔuvsB) | 51.0 ± 6.0 | 30.0 ± 6.0c |
| JFL209-3 (ΔcsnE ΔnpkA) | 65.0 ± 9.0 | 65.0 ± 13.0 |
| JFL209-14 (ΔcsnE ΔuvsB) | 64.0 ± 3.0 | 62.0 ± 6.0 |
Viability was determined as the percentage of colonies on HU-treated plates compared to untreated controls. All the results are the average of determinations from three independent experiments. The data are the average of three repetitions and means ± standard deviation are shown. Statistical differences were determined by ANOVA followed, when significant, by the Newman-Keuls Multiple Comparison Test, using Sigma Stat statistical software (Jandel Scientific). P < 0.05 was considered statistically significant.
Significantly different from the wild type (P < 0.004).
Significantly different from ΔuvsB and ΔcsnD (P < 0.001).
Possible genetic interactions with A. nidulans uvsBATR and npkA:
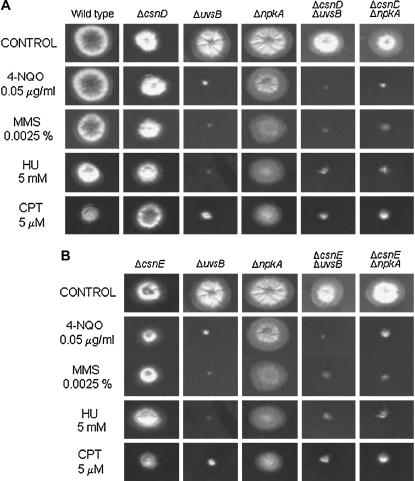
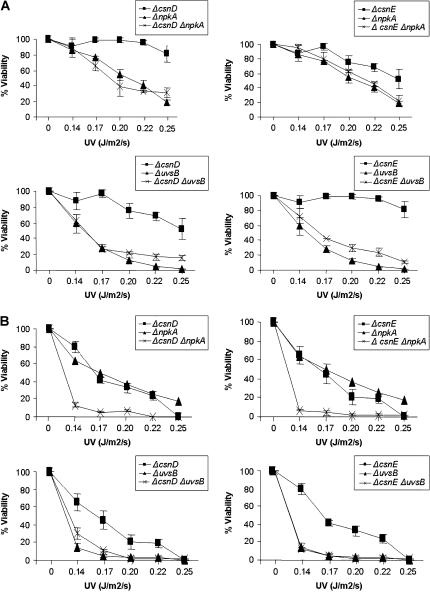
We investigated possible genetic interactions among csnD/csnE and ankAwee1, bimEAPC1, uvsBATR, and the cdc2-related kinase npkA by constructing double mutants with ΔcsnD/ΔcsnE and the other mutants. We were unable to construct double mutants with the bimE7 and ankA and ΔcsnD/ΔcsnE mutants. In both crosses, few cleistothecia of smaller size and without ascospores were produced. The double-mutant strains ΔcsnD ΔnpkA and ΔcsnE ΔnpkA were more sensitive to subinhibitory concentrations of 4-NQO, MMS, HU, and CPT than were the corresponding parental strains (Figure 4). The double-mutant strains ΔcsnD ΔuvsB and ΔcsnE ΔuvsB were inhibited to the same extent as the corresponding parental strain AAH14 (ΔuvsB) when grown in the presence of these DNA-damaging agents (Figure 4). The genetic interaction between csnD/csnE and npkA genes can also be observed when germinating conidia are exposed to UV light (Figure 5B, top). During septation in the presence of MMS, a synergism between uvsBATR and csnE can also be verified, since the double mutant for these genes (JFL209-14) presented an increase in the percentage of septation (60%) in comparison with the corresponding parental strains ΔuvsB and ΔcsnE (22.5 and 36.7%, respectively). Our results suggest that both csnD/csnE and npkA genes genetically interact since there is a synergism in terms of sensitivity to DNA-damaging agents in the corresponding double-mutant strains. Furthermore, ΔuvsB and ΔcsnE also genetically interact during checkpoint-dependent inhibition of septum formation in A. nidulans.
Figure 4.
The ΔnpkA mutant shows synergistic interaction with the csnD/csnE inactivation mutants. (A) Strains AGB152 (wild type), AGB195 (ΔcsnD), AAH14 (ΔuvsBATR), MV3 (ΔnpkA), 195-14 (ΔcsnD ΔuvsB), 195-3 (ΔcsnD ΔnpkA), and (B) AGB209 (ΔcsnE), 209-14 (ΔcsnE ΔuvsB), and 209-3 (ΔcsnE ΔnpkA) were grown for 72 hr at 37° in YUU medium and YUU 0.05 μg/ml 4-NQO, YUU 0.0025% MMS, YUU 5 mm HU, and YUU 5 μm CPT.
Figure 5.
The double mutants ΔcsnD ΔnpkA and ΔcsnE ΔnpkA showed increased sensitivity to UV light. Viability of germlings was scored after exposure to UV light of (A) quiescent and (B) germinating conidiospores. Viability was determined as the percentage of colonies on treated plates compared to untreated controls. The results were expressed by the average of three independent experiments and means are ± standard deviation. Statistical differences were determined by one-way analysis of variance (ANOVA) followed, when significant, by the Newman-Keuls Multiple Comparison Test, using Sigma Stat statistical software (Jandel Scientific, San Rafael, CA). The ΔcsnD ΔnpkA and ΔcsnE ΔnpkA strains were significantly different from ΔcsnD and ΔcsnE (P < 0.01).
We also verified whether the S-phase checkpoints were intact in these double-mutant strains (Tables 5 and 6). As previously shown by Fagundes et al. (2004), the uvsB and npkA inactivation mutants have impaired S-phase checkpoints. During the mitosis assay at both 6 and 100 mm, no genetic interaction among the double-mutant strains was observed. However, the double-mutant ΔcsnD ΔuvsB is impaired in the viability assay at 100 mm (Table 6). Interestingly, the csnD/csnE inactivation mutations are suppressing the absence of the DNA replication checkpoint response in the uvsB inactivation mutant (Table 5), providing additional evidence for genetic interaction between csnD/csnE and uvsB genes.
The ribonucleotide reductase genes have altered mRNA expression in the csnD/csnE inactivation strains:
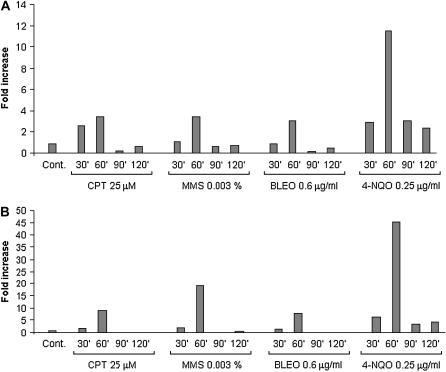
In fission yeast, the COP9 signalosome is required to activate ribonucleotide reductase for DNA synthesis (Nielsen 2003). As a preliminary step to characterize a possible dependence of the ribonucleotide reductase activity on csnD/csnE genes, we identified the A. nidulans ribonucleotide reductase-encoding genes. Like other eukaryotes, A. nidulans has two genes that encode a small (rnrA, which was previously reported by Kraus and Harris 2001; AAG40862) and a large (rnsA) subunit of ribonucleotide reductase (for a review, see Jordan and Reichard 1998). The predicted rnsA and rnrA protein products showed high identity with several ribonucleotide reductases from other eukaryotes (data not shown). We measured the mRNA expression of the rnrA and rnsA genes in the presence of DNA-damaging agents using real-time RT-PCR. As in Figure 2, A. nidulans wild-type GR5 was grown in the absence of any drug and transferred to a specific concentration of a DNA-damaging agent for 30, 60, and 120 min, and then RNA was isolated and analyzed for the expression of these genes (Figure 6). The rnrA gene expression was induced after 60 min growth in the presence of CPT and MMS (∼4 times), BLEO (∼3 times), and 4-NQO (∼12 times) (Figure 6A). Accordingly, the rnsA gene expression was induced after 60 min in the presence of CPT (∼10 times), MMS (∼20 times), BLEO (∼10 times), and 4-NQO (∼45 times) (Figure 6B). These results indicate that rnrA and rnsA genes are induced at transcriptional level by different DNA-damaging agents.
Figure 6.
Fold increase in rnsA and rnrA RNA levels in response to DNA-damaging agents. Mycelia were grown in the absence of any drug, 25 μm CPT, 0.003% MMS, 0.6 μg/ml BLEO, or 0.25 μg/ml 4-NQO for 30, 60, 90, and 120 min. Real-time RT-PCR was the method used to quantify the mRNA. The measured quantity of the (A) rnsA and (B) rnrA mRNA in each of the treated samples of the wild-type GR5 strain was normalized using the CT values obtained for the tubC RNA amplifications run in the same plate. The relative quantitation of rnsA and rnrA and tubulin gene expression was determined by a standard curve (i.e., CT values plotted against the logarithm of the DNA copy number). Results of four sets of experiments were combined for each determination; means are shown. The values represent the number of times that the genes are expressed compared to the wild-type control grown without any drug (represented absolutely as 1.00).
As the next step, we verified rnrA and rnsA gene expression in the ΔcsnD/ΔcsnE mutant and wild-type strains when exposed to CPT. There is an average decrease (∼50%) in rnsA and rnrA gene expression in the ΔcsnD and ΔcsnE mutants, respectively (Figures 7, A and B). We also observed the expression of rnsA and rnrA in the mutants ΔnpkA (MV3), ΔcsnD ΔnpkA (JFL195-3), and ΔcsnE ΔnpkA (JFL209-3) when exposed to CPT (Figures 7, C and D). The ΔnpkA mutant displayed lower levels of rnrA and rnsA gene expression than the wild-type strain did. However, the rnrA and rnsA mRNA expression levels are much more reduced in the double mutants ΔcsnD ΔnpkA and ΔcsnE ΔnpkA than in the corresponding parental strains. These results suggest that the ribonucleotide reductase gene expression upon DNA damage caused by CPT is dependent on the csnD/csnE and npkA genes.
Figure 7.
The rnsA and rnrA genes showed decrease expression when ΔcsnD and ΔcsnE and the double mutants ΔcsnD Δnpka and ΔcsnE ΔnpkA were grown in the presence of 25 μm camptothecin for 30, 60, and 120 min. Real-time RT-PCR was the method used to quantify the mRNA. (A and B) rnsA and rnrA mRNA expression from AGB152 (wild-type strain), AGB195 (ΔcsnD), and AGB209 (ΔcsnE), respectively. (C and D) rnsA and rnrA mRNA expression from MV3 (ΔnpkA), JFL195-3 (ΔcsnD ΔnpkA), and JFL209-3 (ΔcsnE ΔnpkA), respectively. The measured quantity of the rnsA and rnrA mRNA in each of the treated samples was normalized using the CT values obtained for the tubC RNA amplifications run in the same plate. The relative quantitation of csnD/csnE and tubulin gene expression was determined by a standard curve (i.e., CT values plotted against logarithm of the DNA copy number). Results of four sets of experiments were combined for each determination; means ± standard deviations are shown. The values represent the number of times that the genes are expressed compared to the wild-type control grown without any drug (represented absolutely as 1.00).
DISCUSSION
This article investigates the possible involvement of the COP9 signalosome in the DNA damage response in A. nidulans. We examined this idea by assessing several features related to the DNA damage response in this fungus (for a review, see Goldman and Kafer 2004). We observed that growth of the csnD/csnE inactivation mutants is sensitive to DNA-damaging agents and that csnD/csnE mRNA is increased in the presence of these genotoxins. Interestingly, in the csnE gene that encodes the deneddylase activity of the fungal signalosome, this mRNA accumulation is presumably due to increased mRNA stability. Furthermore, there is a decrease in uvsC mRNA expression and elimination of the checkpoint-dependent inhibition of septum formation in the presence of DNA damage in the ΔcsnD/ΔcsnE mutants.
Initially it was thought that in A. thaliana COP9 loss-of-function mutants, the loss of one subunit typically would result in the loss of the entire protein complex, and this could be used to explain the pleiotropic but identical phenotype of these mutants (Schwechheimer and Deng 2001). However, using weaker antisense and cosuppression lines, the reduction of individual CSN subunits brings about partially overlapping but also clearly distinct phenotypes; e.g., while a partial reduction of CSN5 function results in plants with normal flowers, plants with reduced CSN1, CSN3, and CSN6 function have abnormal flower phenotypes (Peng et al. 2001; Schwechheimer et al. 2001; Schwechheimer 2004). Drosophila CSN4 and CSN5 losses of function have common but also distinct developmental phenotypes. CSN4 but not CSN5 mutants display a range of molting defects, while CSN5 but not CSN4 mutant larvae develop melanotic capsules (Oron et al. 2002). Deletion mutants in S. pombe csn1 and csn2 are delayed in S phase and are sensitive to UV light and ionizing radiation. However, mutants of csn3, csn4, and csn5 display neither the phenotypes observed with csn1 and csn2 mutant strains nor any other obvious phenotypes, indicating that distinct CSN subunits may have distinct developmental roles in fission yeast (Mundt et al. 2002). We have observed that ΔcsnD/ΔcsnE mutations are epistatic for most of the phenotypes related to DNA damage that were investigated in this work. Busch et al. (2003) have established csnD/csnE genes as regulators of sexual development in A. nidulans. The deletion of each of these genes resulted in indistinguishable phenotypes, suggesting that they exhibit the expected epistatic behavior. However, there are circumstances in our work where they do not demonstrate the same phenotypes, for instance: (i) csnD/csnE mRNA expression is not synchronized since csnE expression seems to lag behind that of csnD; (ii) the ΔcsnE inactivation mutant does not affect rnsA mRNA expression while the ΔcsnD mutation does not really affect rnrA mRNA expression; (iii) the ΔcsnE mutation enhances the septation of the ΔuvsB mutant during checkpoint-dependent inhibition of septum formation, whereas the ΔcsnD mutation does not; and (iv) the ΔcsnD/ΔcsnE mutations have suppression effects on the ΔuvsB mutation in the viability and mitosis assays, respectively. Hence, despite its epistasis, specific biological functions can be attributed to individual CSN subunits. We are currently constructing a double-mutant ΔcsnD ΔcsnE strain aiming to study it for epistasis under some of the conditions where the single csnD/csnE mutants showed different phenotypes.
We also constructed double mutants with cnsD-E inactivation and deletion mutants of two genes, uvsBATR and npkA, which are involved in several aspects of the DNA damage response in A. nidulans. ATM/ATR are members of the family of phosphoinositide 3-kinase-related kinases and key regulators of the DNA damage response (Caspari and Carr 1999; Shiloh 2001). The ATR pathway can respond to agents that interfere with the function of replication forks, such as HU, UV light, and DNA-alkylating agents such as MMS (Nyberg et al. 2002; Osborn et al. 2002). The ATM/ATR kinases phosphorylate and activate signal transduction pathways that ultimately interface with the Cdk/cyclin machinery (Abraham 2001). They trigger responses that promote the maintenance of genome integrity by phosphorylating multiple target proteins. A. nidulans UvsBATR is a member of the family of ATM/ATR kinases and functions as the central regulator of the A. nidulans DNA damage response (De Souza et al. 1999; Hofmann and Harris 2000; Fagundes et al. 2004). NpkA is a cdc2-related kinase that together with NimXCdc2 and BimEAPC1 monitors S-phase progression and/or recovery in response to DNA damage. We have observed that the double-mutant strains ΔcsnD ΔnpkA and ΔcsnE ΔnpkA were more sensitive to 4-NQO, MMS, HU, and CPT than the corresponding parental strains. Interestingly, the enhanced UV sensitivity of germinating conidia from the double mutants ΔcsnD/ΔcsnE ΔnpkA, compared to the lack of interaction in quiescent conidia, suggests a possible role in checkpoint responses since there is no need to arrest the cell cycle for quiescent conidia. These data strongly support the notion that the signalosome may work in parallel with npkA to regulate DNA damage checkpoint responses. Another example of either gain or loss of CSN functions can perturb normal cell progression is the cell cycle inhibitor p27KIP1, which is subject to regulation by CSN-mediated deneddylation through SCFSKP2 via deneddylation and nuclear export (Tomoda et al. 1999; Yang et al. 2002; Wei and Deng 2003).
In A. nidulans, the DNA damage checkpoint regulates septation by modulating the activity of NimXcdc2 (Harris and Kraus 1998; De Souza et al. 1999). Septum formation is triggered by high levels of NimXcdc2 activity and is inhibited by DNA damage in a checkpoint-dependent manner (Harris and Kraus 1998). Harris and Kraus (1998) showed that mutations in uvsBATR abolish the cell cycle delay and inhibition of septum formation triggered by the exposure of predivisional hyphae to DNA-damaging agents. We detected increased septation levels in the csnD/csnE inactivation mutants in the presence of DNA damage. Furthermore, ΔuvsB and ΔcsnE also genetically interact during checkpoint-dependent inhibition of septum formation in A. nidulans. Our results suggest that regulation of checkpoint-dependent inhibition of septum formation in A. nidulans is dependent not only on the uvsBATR gene but also on the CSN complex. Moreover, we have also observed that csnD/csnE inactivation mutations suppress the absence of DNA replication checkpoint response in the uvsB inactivation mutant. Thus, it is possible that the signalosome and UvsB function in parallel pathways to regulate the septation checkpoint, whereas they function in the same pathway to control mitotic checkpoints.
CSN is involved in multiple aspects of cell cycle and checkpoint control (for a review, see Wei and Deng 2003). Mutations in Drosophila CSN5 cause activation of mei-41-mediated meiotic DNA damage checkpoint during oogenesis (Doronkin et al. 2002). The fission yeast csn1 and csn2 mutants are synthetically lethal when combined with checkpoint pathway mutants such as rad3, chk-1, cds-1, and cdc2.w and more sensitive to gamma and UV irradiation (Mundt et al. 2002). In S. pombe, csn1 and csn2 are required for proper S-phase progression (Mundt et al. 2002). Further study has revealed that the S-phase delay observed in S. pombe csn1 and csn2 mutants is due at least in part to the misregulation of RNR, a key enzyme in the biosynthesis of deoxyribonucleotides (Liu et al. 2003). In S. pombe, RNR is composed of two small subunits (Suc22) and two large subunits (Cdc22). Activation of RNR involves nuclear export of Suc22, a process inhibited by a small cell cycle inhibitor, Spd1 (S phase delayed), whose level transiently declines in S phase and in response to DNA damage (Liu et al. 2003). Deletion of csn1 leads to Spd1 accumulation owing to a defect in proteolysis. This prevents Suc22 nuclear export and ultimately leads to S-phase delay and DNA damage sensitivity. The ΔcsnE strain showed defects in the mitosis assay (6 mm of HU). These results suggest a possible engagement of the CSN complex in A. nidulans S-phase progression and/or recovery.
As an initial step toward understanding the observed S-phase checkpoint deficiencies and npkA interaction with the csnD/csnE inactivation mutants, we identified the A. nidulans RNR genes. As in most eukaryotes (Jordan and Reichard 1998), A. nidulans also has two RNR-encoding genes, rnrA and rnsA, which encode the small and large subunits, respectively. These two genes have increased mRNA accumulation when A. nidulans is grown in the presence of DNA-damaging agents. Interestingly, the mRNA expression of these genes is reduced in both ΔcsnD/ΔcsnE and the double mutants ΔcsnD Δnpka and ΔcsnE ΔnpkA upon DNA damage caused by CPT. These results suggest that the RNR genes could also play a role in the CSN-mediated DNA damage response in A. nidulans. Additionally, the defect in the ribonucleotide reductase expression in the double mutants ΔcsnD Δnpka and ΔcsnE ΔnpkA reinforces the hypothesis that it may act in a common checkpoint pathway that also regulates the ribonucleotide reductase gene expression.
In conclusion, our data are consistent with the possibility that the CSN complex is involved in cell cycle and checkpoint response upon DNA damage in A. nidulans. NpkA and UvsBATR appear to play an important role in signalosome biological interactions because the corresponding double mutants with ΔcsnD and ΔcsnE have deficiencies in several aspects of DNA damage response.
Acknowledgments
This research was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo and the Conselho Nacional de Desenvolvimento Científico e Tecnológico, São Paulo, Brazil (to G.H.G.) and the Deutsche Forschungsgemeinschaft (German Research Council) to G.H.B.
References
- Abraham, R. T., 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15: 2177–2196. [DOI] [PubMed] [Google Scholar]
- Aist, J. R., and N. R. Morris, 1999. Mitosis in filamentous fungi: how we got where we are. Fungal Genet. Biol. 27: 1–25. [DOI] [PubMed] [Google Scholar]
- Baumeister, W., J. Walz, F. Zuhl and E. Seemuller, 1998. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92: 367–380. [DOI] [PubMed] [Google Scholar]
- Bech-Otschir, D., R. Kraft, X. Huang, P. Henklein, B. Kapelari et al., 2001. COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J. 20: 1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech-Otschir, D., M. Seeger and W. Dubiel, 2002. The COP9 signalosome: at the interface between signal transduction and ubiquitin-dependent proteolysis. J. Cell Sci. 115: 467–473. [DOI] [PubMed] [Google Scholar]
- Bergen, L. G., and N. R. Morris, 1983. Kinetics of the nuclear division cycle of Aspergillus nidulans. J. Bacteriol. 156: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, M. M, 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Bruschi, G. C. M., C. C. de Souza, M. R. Z. K. Fagundes, M. A. C. Dani, M. H. S. Goldman et al., 2001. Sensitivity to camptothecin in Aspergillus nidulans identifies a novel gene, scaA, related to the cellular DNA damage response. Mol. Genet. Genomics 265: 264–275. [DOI] [PubMed] [Google Scholar]
- Busch, S., S. E. Eckert, S. Krappmann and G. H. Braus, 2003. The COP9 signalosome is an essential regulator of development in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 49: 717–730. [DOI] [PubMed] [Google Scholar]
- Caspari, T., and A. M. Carr, 1999. DNA structure checkpoint pathways in Schizosaccharomyces pombe. Biochimie 81: 173–181. [DOI] [PubMed] [Google Scholar]
- Caspari, T., J. M. Murray and A. M. Carr, 2002. Cdc2-cyclin B kinase activity links Crb2 and Rqh1-topoisomerase III. Genes Dev. 16: 1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamovitz, D. A., N. Wei, M. T. Osterlund, A. G. von Arnim, J. M. Staub et al., 1996. The COP9 complex, a novel multisubunit nuclear regulator involved in light control of a plant developmental switch. Cell 86: 115–121. [DOI] [PubMed] [Google Scholar]
- Cope, G. A., and R. J. Deshaies, 2003. COP9 signalosome: a multifunctional regulator of SCF and other cullin-based ubiquitin ligases. Cell 114: 663–671. [DOI] [PubMed] [Google Scholar]
- Cope, G. A., G. S. Suh, L. Aravind, S. E. Schwarz, S. L. Zipursky et al., 2002. Role of predicted metalloprotease motif of Jab1/Csn5 in cleavage of NEDD8 from CUL1. Science 15: 608–661. [DOI] [PubMed] [Google Scholar]
- Desany, B. A., A. A. Alcasabas, J. B. Bachant and S.J. Elledge, 1998. Recovery from DNA replicational stress is the essential function of the S phase checkpoint pathway. Genes Dev. 12: 2956–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza, C. P. C., X. S. Ye and S.A. Osmani, 1999. Checkpoint defects leading to premature mitosis also cause endoreplication of DNA in Aspergillus nidulans. Mol. Biol. Cell 10: 3661–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronkin, S., I. Djagaeva and S.K. Beckendorf, 2002. CSN5/Jab1 mutations affect axis formation in the Drosophila oocyte by activating a meiotic checkpoint. Development 129: 5053–5064. [DOI] [PubMed] [Google Scholar]
- Eckert, S. E., E. Kübler, B. Hoffmann and G. H. Braus, 2000. The tryptophan synthase-encoding trpB gene of Aspergillus nidulans is regulated by the cross-pathway control system. Mol. Gen. Genet. 263: 867–876. [DOI] [PubMed] [Google Scholar]
- Fagundes, M. R., L. Fernandes, M. Savoldi, S. D. Harris, M. H. S. Goldman et al., 2003. Identification of a topoisomerase I mutant, scsA1, as an extragenic suppressor of a mutation in scaANBS1, the apparent homolog of human nibrin in Aspergillus nidulans. Genetics 164: 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes, M. R., J. F. Lima, M. Savoldi, I. Malavazi, R. E. Larson et al., 2004. The Aspergillus nidulans npkA gene encodes a Cdc2-related kinase that genetically interacts with the UvsBATR kinase. Genetics 167: 1629–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, S., and A. M. Weissman, 2004. A field guide to ubiquitylation. Cell. Mol. Life Sci. 61: 1546–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freilich, S., E. Oron, Y. Kapp, Y. Nevo-Caspi, S. Orgad et al., 1999. The COP9 signalosome is essential for development of Drosophila melanogaster. Curr. Biol. 9: 1187–1190. [DOI] [PubMed] [Google Scholar]
- Gasior, S. L., H. Olivares, U. Ear, D. M. Hari, R. Weichselaum et al., 2001. Assembly of RecA-like recombinases: distinct roles for mediator proteins in mitosis and meiosis. Proc. Natl. Acad. Sci. USA 98: 8411–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman, M. H., D. M. Rubin, O. Coux, I. Wefes, G. Pfeifer et al., 1998. A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell 94: 615–623. [DOI] [PubMed] [Google Scholar]
- Goldman, G. H., and E. Kafer, 2004. Aspergillus nidulans as a model system to characterize the DNA damage response in eukaryotes. Fungal Genet. Biol. 41: 428–442. [DOI] [PubMed] [Google Scholar]
- Goldman, G. H., S. L. McGuire and S. D. Harris, 2002. The DNA damage response in filamentous fungi. Fungal Genet. Biol. 35: 183–195. [DOI] [PubMed] [Google Scholar]
- Harris, S. D., and P. R. Kraus, 1998. Regulation of septum formation in Aspergillus nidulans by a DNA damage checkpoint pathway. Genetics 148: 1055–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, A. F., and S. D. Harris, 2000. The Aspergillus nidulans uvsB gene encodes an ATM-related kinase required for multiple facets of the DNA damage response. Genetics 154: 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, A., and P. Reichard, 1998. Ribonucleotide reductases. Annu. Rev. Biochem. 67: 71–98. [DOI] [PubMed] [Google Scholar]
- Kafer, E., 1977. Meiotic and mitotic recombination in Aspergilllus and its chromosomal aberrations. Adv. Genet. 19: 33–131. [DOI] [PubMed] [Google Scholar]
- Kafer, E., and G. S. May, 1998. Toward repair pathways in Aspergillus nidulans, pp. 477–502 in DNA Damage and Repair, Vol. 1: DNA Repair in Prokaryotes and Lower Eukaryotes, edited by J. A. Nickoloff and M. F. Hoekstra. Humana Press, Totowa, NJ.
- Kim, T., K. Hofmann, A. G. von Arnim and D. A. Chamovitz, 2001. PCI complexes: pretty complex interactions in diverse signaling pathways. Trend Plant Sci. 6: 379–386. [DOI] [PubMed] [Google Scholar]
- Kraus, P. R., and S. D. Harris, 2001. The Aspergillus nidulans snt genes are required for the regulation of septum formation and cell cycle checkpoints. Genetics 159: 557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., X. Liu and M. Ascoli, 2000. p38JAB1 binds to the intracellular precursor of the lutropi/choriogonadotropin receptor and promotes its degradation. J. Biol. Chem. 275: 13386–13393. [DOI] [PubMed] [Google Scholar]
- Liu, C., K. A. Powell, K. Mundt, L. Wu, A. M. Carr et al., 2003. Cop9/signalosome subunits and Pcu4 regulate ribonucleotide reductase by both checkpoint-dependent and -independent mechanisms. Genes Dev. 17: 1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyapina, S., G. Cope, A. Shevchenko, G. Serino, T. Tsuge et al., 2001. Promotion of NEDD-CUL1 conjugate cleavage by COP9 signalosome. Science 292: 1382–1385. [DOI] [PubMed] [Google Scholar]
- Maytal-Kivity, V., R. Piran, E. Pick, K. Hofmann and M. H. Glickman, 2002. COP9 signalosome components play a role in the mating pheromone response of S. cerevisiae. EMBO Rep. 13: 1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill, B. J., and C. Holm, 1999. A requirement for recombinational repair in Saccharomyces cerevisiae is caused by DNA replication defects of mec1 mutants. Genetics 153: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J. H., 1972. Experiments in Molecular Genetics, pp. 352–355. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Mundt, K. E., C. Liu and A. M. Carr, 2002. Deletion mutants in COP9/signalosome subunits in fission yeast Schizosaccharomyces pombe display distinct phenotypes. Mol. Biol. Cell 13: 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, O., 2003. COP9 signalosome: a provider of DNA building blocks. Curr. Biol. 13: R565–R567. [DOI] [PubMed] [Google Scholar]
- Nyberg, K. A., R. J. Michelson, C. W. Putnam and T. A. Weinert, 2002. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36: 617–656. [DOI] [PubMed] [Google Scholar]
- Oron, E., M. Mannervik, S. Rencus, O. Harari-Steinberg, S. Neumann-Siberberg et al., 2002. COP9 signalosome subunits 4 and 5 regulate multiple pleiotropic pathways in Drosophila melanogaster. Development 129: 4399–4409. [DOI] [PubMed] [Google Scholar]
- Osborn, A. J., S. J. Elledge and L. Zou, 2002. Checking on the fork: the DNA-replication stress-response pathway. Trends Cell Biol. 12: 509–516. [DOI] [PubMed] [Google Scholar]
- Osmani, S. A., and P. M. Mirabito, 2004. The early impact of genetics on our understanding of cell cycle regulation in Aspergillus nidulans. Fungal Genet. Biol. 41: 401–410. [DOI] [PubMed] [Google Scholar]
- Osmani, S. A., and X. S. Ye, 1996. Cell cycle regulation in Aspergillus nidulans by two protein kinases. Biochem. J. 317: 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani, A. H., S. L. McGuire and S. A. Osmani, 1991. Parallel activation of the NIMA and p34cdc2 cell cycle-regulated protein kinases is required to initiate mitosis in A. nidulans. Cell 67: 283–291. [DOI] [PubMed] [Google Scholar]
- Osterlund, M. T., C. S. Hardtke, N. Wei and X-W. Deng, 2000. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 662–666. [DOI] [PubMed] [Google Scholar]
- Peng, Z., G. Serino and X-W Deng, 2001. A role of Arabidopsis COP9 signalosome in multifaceted developmental processes revealed by the characterization of its subunit 3. Development 128: 4277–4288. [DOI] [PubMed] [Google Scholar]
- Pollmann, C., X. Huang, J. Mall, D. Besch-Otschir, M. Naumann et al., 2001. The constitutive photomorphogenesis 9 signalosome directs vascular endothelial growth factor production in tumor cells. Cancer Res. 61: 8416–8421. [PubMed] [Google Scholar]
- Punt, P. J., M. A. Dingemanse, A. Kuyvenhoven, R. D. M Soede, P. H. Powels et al., 1990. Functional elements in the promotor region of the Aspergillus nidulans gpda gene encoding glycer-aldehyde-3-phosphate dehydrogenase. Gene 93: 101–109. [DOI] [PubMed] [Google Scholar]
- Schwechheimer, C., 2004. The COP9 signalosome (CSN): an evolutionary conserved proteolysis regulator in eukaryotic development. Biochim. Biophys. Acta 1695: 45–54. [DOI] [PubMed] [Google Scholar]
- Schwechheimer, C., and X. W. Deng, 2001. COP9 signalosome revisited: a novel mediator of protein degradation. Trends Cell Biol. 11: 420–426. [DOI] [PubMed] [Google Scholar]
- Schwechheimer, C., G. Serino, J. Callis, W. L. Crosby, S. Lyapina et al., 2001. Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 292: 1379–1382. [DOI] [PubMed] [Google Scholar]
- Semighini, C. P., M. Marins, M. H. S. Goldman and G. H. Goldman, 2002. Quantitative analysis of the relative transcript levels of ABC transporter Atr genes in Aspergillus nidulans by real-time reverse transcripition-PCR assay. Appl. Environ. Microbiol. 68: 1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semighini, C. P., M. R. Von Zeska Kress Fagundes, J. C. Ferreira, R. C. Pascon, M. H. S. Goldman et al., 2003. Different roles of the Mre11 complex in the DNA damage response in Aspergillus nidulans. Mol. Microbiol. 48: 1693–1709. [DOI] [PubMed] [Google Scholar]
- Shiloh, Y., 2001. ATM and ATR: networking cellular responses to DNA damage. Curr. Opin. Genet. Dev. 11: 71–77. [DOI] [PubMed] [Google Scholar]
- Southern, E. M., 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98: 503–517. [DOI] [PubMed] [Google Scholar]
- Sun, Y., M. P. Wilson and P. W. Majerus, 2002. Inositol1,3,4-triphosphate associates with the COP9 signalosome by binding to CSN1. J. Biol. Chem. 24: 45759–45764. [DOI] [PubMed] [Google Scholar]
- Tomoda, K., Y. Bubota and J-Y. Kato, 1999. Degradation of the cyclin-dependent-kinase inhibitor p27KIP1 is instigated by JAB1. Nature 398: 160–165. [DOI] [PubMed] [Google Scholar]
- Van Gorcom, R. F. M., P. J. Punt, P. H. Pouwels and C. A. M. J. J. van den Hondel, 1986. A system for the analysis of expression signals in Aspergillus. Gene 48: 211–217. [DOI] [PubMed] [Google Scholar]
- Van Heemst, D., K. Swart, E. F. Holub, R. van Dijk, H. H. Offenberg et al., 1997. Cloning, sequencing, disruption, and phenotypic analysis of uvsC, an Aspergillus nidulans homologue of yeast RAD51. Mol. Gen. Genet. 254: 654–664. [DOI] [PubMed] [Google Scholar]
- Wee, S., B. Heltfeld, W. Dubiel and D. A. Wolf, 2002. Conservation of the COP9/signalosome in budding yeast. BMC Genet. 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, N., and X-W. Deng, 1999. Making sense of the COP9 signalosome. A regulatory protein complex conserved from Arabidopsis to human. Trends Genet. 15: 98–103. [DOI] [PubMed] [Google Scholar]
- Wei, N., and X-W. Deng, 2003. The COP9 signalosome. Annu. Rev. Cell Dev. Biol. 19: 261–286. [DOI] [PubMed] [Google Scholar]
- Wei, N., D. A. Chamovitz and X. W. Deng, 1994. Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development. Cell 78: 117–124. [DOI] [PubMed] [Google Scholar]
- Yang, X., S. Menon, K. Lykke-Andersen, T. Tsuge, Di Xiao et al., 2002. The COP9 signalosome inhibits p27 (kip1) degradation and impedes G1-S phase progression via deneddylation of SCF Cul1. Curr. Biol. 12: 667–672. [DOI] [PubMed] [Google Scholar]
- Ye, X. S., R. R. Fincher, A. Tang and S. A. Osmani, 1997. The G2/M DNA damage checkpoint inhibits mitosis through Tyr15 phosphorylation of p34cdc2 in Aspergillus nidulans. EMBO J. 16: 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C., V. Seibert, R. Geyer, E. Rhee, S. Lyapina et al., 2001. The fission yeast COP9/signalosome is involved in cullin modification by ubiquitin-related Ned8p. BMC Biochem. 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]