Abstract
Smk1 is a meiosis-specific MAPK homolog in Saccharomyces cerevisiae that regulates the postmeiotic program of spore formation. Similar to other MAPKs, it is activated via phosphorylation of the T-X-Y motif in its regulatory loop, but the signals controlling Smk1 activation have not been defined. Here we show that Ama1, a meiosis-specific activator of the anaphase-promoting complex/cyclosome (APC/C), promotes Smk1 activation during meiosis. A weakened allele of CDC28 suppresses the sporulation defect of an ama1 null strain and increases the activation state of Smk1. The function of Ama1 in regulating Smk1 is independent of the FEAR network, which promotes exit from mitosis and exit from meiosis I through the Cdc14 phosphatase. The data indicate that Cdc28 and Ama1 function in a pathway to trigger Smk1-dependent steps in spore morphogenesis. We propose that this novel mechanism for controlling MAPK activation plays a role in coupling the completion of meiosis II to gamete formation.
MEIOSIS is the program used by sexually reproducing organisms to produce haploids from diploid precursors. The completion of meiosis is coupled to differentiation programs that produce gametes, which are capable of sexual fusion. Little is known about how meiosis and gametogenesis are linked. In the yeast Saccharomyces cerevisiae, meiosis is coupled to spore formation.
One mechanism used by yeast cells to regulate meiosis and spore formation is a transcriptional cascade that leads to the accumulation of gene products in three major regulatory stages termed early, middle, and late (Chu et al. 1998; Primig et al. 2000). The cascade is initiated by the Ime1 transcription factor whose expression is limited to diploid cells where it can be induced by starvation signals (Kassir et al. 1988). Ime1 functions at early gene promoters, which are expressed as premeiotic DNA replication is occurring and as synapsis and genetic recombination are taking place in prophase (Honigberg and Purnapatre 2003). Middle gene expression is activated by the meiosis-specific Ndt80 transcription factor, which binds to DNA sequences termed middle sporulation elements (MSEs) in middle promoters (Chu and Herskowitz 1998). Sum1, a repressor protein that binds to a subset of MSEs and competes with Ndt80 for occupancy at key promoters, also controls middle-gene expression (Xie et al. 1999; Pierce et al. 2003). The NDT80 promoter is regulated by competition between Sum1 and Ndt80. These interactions control a positive feedback loop leading to high-level Ndt80 expression as cells exit prophase and enter the meiotic divisions (Pak and Segall 2002a). Some middle genes, such as the B-type cyclins, promote chromosome segregation, while others are required for spore formation. Thus, the transcriptional cascade of sporulation assures that gene products required for the meiotic divisions and spore formation accumulate around the same time. Signaling pathways play key roles in assuring that meiotic events occur in the proper order in response to internal cues. A role for signaling pathways and the cell cycle machinery in coupling meiosis to spore formation has not been described.
During mitosis, key cell cycle events are controlled through the Cdc28 cyclin-dependent kinase (CDK) (Mendenhall and Hodge 1998). Cdc28 also controls key steps in meiosis, including DNA replication and the nuclear divisions (Shuster and Byers 1989; Benjamin et al. 2003). However, Cdc28 is regulated differently in meiosis than in mitosis, in part through the transcriptional program of sporulation. Another family of protein kinases that can regulate mitotic and meiotic events are mitogen-activated protein kinases (MAPKs), which are activated by dual phosphorylation of T-X-Y motifs in their activation loops through conserved kinase cascades (Widmann et al. 1999). In mitosis, MAPKs couple to a variety of receptors and control the cell cycle in response to mitogenic/differentiation signals by phosphorylating transcription factors and other effector molecules. MAPK targets can control the cell cycle, at least in part, by regulating the expression of genes encoding CDK inhibitors and activators. Little is known about how CDKs might, in turn, regulate MAPK pathways to coordinate differentiation with the cell cycle.
In the budding yeast there are five MAPK homologs. Four of these couple to defined extracellular signals. The Fus3, Kss1, Mpk1, and Hog1 MAPKs respond to mating pheromone, nutritional, cell wall integrity, and osmotic signals, respectively, to induce responses that are appropriate to the extracellular signals (Widmann et al. 1999). The fifth MAPK homolog in yeast is Smk1, which is expressed only during sporulation and regulates the postmeiotic program of spore formation (Krisak et al. 1994). In smk1 null mutants, meiosis I (MI) and meiosis II (MII) are completed on schedule but the multilayered spore wall that normally surrounds haploid products is assembled in a deregulated fashion. Different abnormal spore wall patterns are observed even within a single smk1-null ascus with layers missing, inverted, or otherwise abnormal in appearance. In contrast, smk1 hypomorphs block at distinct steps in the spore assembly pathway and allelic dosage studies indicate that increasing Smk1 activity levels are required to complete successively later steps in the pathway (Wagner et al. 1999). These phenotypes suggest that the regulated activity of Smk1 plays a central role in coordinating multiple steps in the spore morphogenesis program. Smk1 contains a MAPK activation (T-X-Y) motif that is phosphorylated when Smk1 becomes active and mutants lacking phosphorylatable amino acids at these positions show an smk1-null phenotype, indicating that Smk1 is controlled by upstream kinases (Schaber et al. 2002).
Despite the similarities between Smk1 and other MAPKs, Smk1 is unique in several key respects. One unique property of Smk1 is that its expression is tightly regulated as a middle sporulation-specific gene. Another difference between Smk1 and other MAPKs is that, to date, there is no evidence that Smk1 is activated through a hierarchical MAPKKK → MAPKK → MAPK cascade. Moreover, homozygous diploids lacking known MAPKKK (Stell) or MAPKK (Ste7, Pbs2, Mkk1, Mkk2) family members form spores and activate Smk1, indicating that key kinases that are utilized in the Fus3, Kss1, Hog1, and Mpk1 pathways are not uniquely required for Smk1 activation (our unpublished data). The only known protein kinase activator of Smk1 is the CDK-activating kinase Cak1 (Wagner et al. 1997; Schaber et al. 2002), which is also required to activate Cdc28 by phosphorylation. Another difference between Smk1 and the other yeast MAPKs is that Smk1 does not appear to directly couple to extracellular signals since it functions after the time when nutritional signals control the program. Instead, Smk1 is more likely to be controlled by internal signals generated during meiotic progression.
One internal signal that could trigger the phosphorylation of Smk1's activation loop is the completion of meiosis. Regulatory pathways that signal the completion of chromosome segregation include the Cdc14 early release network (FEAR) and the mitotic exit network (MEN) that couple completion of chromosome segregation in mitosis and meiosis I to key cell cycle events such as disassembly of the spindle apparatus (D'Amours and Amon 2004). The FEAR and MEN pathways promote the transient and then sustained release, respectively, of the Cdc14 phosphatase from the nucleolus where an inactivating binding partner named Cfi1/Net1 holds it. Cdc14 acts in opposition to Cdc28 by removing key phospho-modifications to cell cycle regulatory proteins that, in turn, downregulate Cdc28 itself. In meiosis, additional regulatory features are superimposed on the FEAR and MEN network to enable two consecutive rounds of chromosome segregation to occur without an intervening S phase (Buonomo et al. 2003; Marston et al. 2003).
The anaphase-promoting complex/cyclosome (APC/C) controls chromosome segregation by promoting the ubiquitylation and destruction of key cell cycle regulatory substrates (Harper et al. 2002). The APC/C is regulated by Cdc20, a specificity (bridging) protein that targets substrates such as the Pds1 securin for ubiquitylation to trigger chromosome segregation. Cdc20 also targets B-type cyclins for ubiquitylation. In addition, a homolog of Cdc20 named Cdh1 (Hct1) can ubiquitylate B-type cyclins when Cdh1 is dephosphorylated by Cdc14. Active Cdh1 ubiquitylates Cdc20, assuring that a single targeting form of the APC/C is present at any given phase of the cell cycle and reinforcing exit from mitosis and progression into G1.
The S. cerevisiae AMA1 gene was first identified as a Cdc20 family member that is transcribed as a middle sporulation-specific gene and spliced in a pathway that requires the MER1 meiosis-specific splicing factor (Cooper et al. 2000). In some genetic backgrounds ama1 mutants show delayed and reduced MI (Cooper et al. 2000). However, an ama1 mutant in the hypersporulating SK1 genetic background completes meiosis normally (Rabitsch et al. 2001; Oelschlaegel et al. 2005; Penkner et al. 2005). ama1 mutants fail to form spores in all genetic backgrounds tested. In the SK1 background, ama1 mutants block shortly after completion of meiosis II during prospore membrane closure, the cellularization event defined as the earliest stage in the spore morphogenesis pathway (Coluccio et al. 2004). Ama1 is capable of targeting proteins, including Clb1 for ubiquitylation (Cooper et al. 2000). However, an APC/C-associated protein named Mnd2 inhibits Ama1 (Oelschlaegel et al. 2005; Penkner et al. 2005). Thus, cells lacking Mnd2 prematurely ubiquitylate and degrade Pds1 when Ama1 is first produced in meiotic prophase, leading to premature chromosome segregation and a block to sporulation. Mnd2 disappears from cells at the metaphase-to-anaphase transition of MII. It has been proposed that Cdc28 promotes Mnd2's repression of Ama1 and that this repression is relieved at the completion of MII (Oelschlaegel et al. 2005; Penkner et al. 2005). The fission yeast Schizosaccharomyces pombe also contains a Cdc20 family member named Mfr1/Fzr1 that is expressed exclusively in meiosis (Asakawa et al. 2001; Blanco et al. 2001). Strains lacking Mfr1 complete MI and MII normally but fail to make spores. It has been proposed that Mfr1 targets the Cdc13 B-type cyclin for ubiquitylation and that the subsequent destruction of Cdc13 and downregulation of CDK activity is required for spore formation.
In this study we screened through a collection of sporulation-defective mutants to identify regulators of the Smk1 pathway. These experiments demonstrate that Ama1 is required for Smk1 activation. Our data indicate that Cdc28 inhibits Smk1 during meiosis and that Ama1 is required to relieve this inhibition. These data define a novel meiosis-specific pathway that couples activation of a MAPK to completion of meiosis.
MATERIALS AND METHODS
Yeast strains, plasmids, and culture conditions:
The diploid deletion strains used in the Smk1 activation screen shown were obtained from Open Biosystems. For the screen, homozygous diploids lacking the indicated genes (Table 2) were cotransformed with pSL1, a CEN-based URA3-selectable plasmid containing the SMK1 gene fused to three copies of the hemagglutinin epitope (Schaber et al. 2002) and with pS303 (identical to Yep351-IME1), a 2μ-based LEU2-selectable plasmid containing IME1 (Honigberg and Lee 1998). Transformants harboring both plasmids were selected on SD-LEU-URA, expanded in liquid selective medium, and inoculated in YPD for overnight growth to mid-log phase. Cells were maintained in log phase throughout since the high-copy pS303 IME1 plasmid can cause premature entry of stationary phase cells into meiosis. To induce sporulation, cells were harvested by centrifugation, washed once, and resuspended at 1 × 107 cells/ml in 2% potassium acetate containing trace amounts (0.05%) of glucose. Cells were collected at 24 hr postinduction, and extracts were prepared for immunoblot analysis (see below).
TABLE 2.
Smk1 expression/activation in sporulation-defective mutants
| Class I (undetectable) | Class II (wild type) | Class III (activation defective) | |
|---|---|---|---|
| apg1 | bub3 | ssn3 | ama1 (spo70) |
| bim1 | dmc1 | ssn8 | ssp2 |
| bre1 | doa1 | sso1 | |
| ctk1 | fks1 | ssp1 | |
| ctk3 | gip1 | swm1 | |
| dep1 | ids2 | ubi4 | |
| erv14 | isc1 | vam6 | |
| hop2 | mck1 | yal068c | |
| ime2 | mei5 | ycr105w | |
| mnd2 | mpc54 | ydl041w | |
| mum2 | pcl1 | ydr049w | |
| nem1 | ndt80 | ydr070c | |
| pkh2 | nat1 | ydr117c | |
| rad6 | rim15 | ygl020c | |
| rim4 | rim101 | ykr089c | |
| rec8 | slk19 | ylr021w | |
| rim11 | sma1 | ylr235c | |
| sac7 | sma2 | ymr010c | |
| slg1 | spo7 | ymr158w-a | |
| spo1 | spo11 | ynl296w | |
| spo19 | spo12 | ynl170w | |
| spt3 | spo14 | ynl196c | |
| ubc8 | spo16 | yor333c | |
| vac8 | spo20 | zip1 | |
| vam7 | spo21 | ||
| vps30 | spo71 | ||
| ycl010c | spo73 | ||
| ydr126w | spo74 | ||
| yhl0232c | spo75 | ||
| ypt7 | spo77 | ||
All of the experiments except for the Smk1 activation screen shown in Figure 1 were done in the SK1-genetic background (Table 1). The ALY60 SMK1-HA∷kanMX6 strain was derived by standard genetic methods from strain DBY3, which has previously been described (Schaber et al. 2002). The ama1∷kanMX6 allele removed the entire AMA1 open reading frame and was generated by transformation using PCR products as described (Longtine et al. 1998). The cdc28-4 strain was generated using an integrating plasmid (pTB1) that was constructed by subcloning a BamHI/XhoI restriction endonuclease fragment containing the cdc28-4 allele (Lorincz and Reed 1986) from pRS413/cdc28-4 (obtained from Steven Kron, University of Chicago) into these same restriction endonuclease sites in pRS406. A haploid derivative of ALY60 (Table 1) was transformed with pTB1 DNA that had been digested with AflII to direct intergration to the CDC28 locus, and Ura+ transformants were selected and subsequently counterselected using 5-fluoroorotic acid. Resistant colonies with a temperature-sensitive growth phenotype were identified and the mutation was confirmed by sequence analysis of DNA fragments containing the CDC28 open reading frame that were generated by PCR. The spo13 and spo11 strains listed in Table 1 were generated by crossing SK1 strains LNY66 (obtained from Lenore Neigeborne, Rutgers University) and NKY644 (obtained from Nancy Kleckner, Harvard University), respectively, by haploid segregants of ALY60. The cdc14-1, slk19, and spo12 strains listed in Table 1 were generated by crossing SK1 strains 5161, 4233, and 1504, respectively (obtained from Angelika Amon, MIT), with haploid segregants of ALY60. Haploid strains containing multiple kan-marked mutations were generated using standard genetic methods and confirmed by PCR. Sporulation of SK1 strains was carried out starting with logarithmically growing cultures prepared by growing cells overnight to a density of 107 cells/ml (log phase) in YEPA (1% yeast extract, 2% peptone, 2% potassium acetate), collecting cells by centrifugation, washing in SM (2% potassium acetate, 10 μg/ml adenine, 5 μg/ml histidine, 30 μg/ml leucine, 7.5 μg/ml lysine, 10 μg/ml tryptophan, 5 μg/ml uracil), and resuspending cells at 4 × 107 cells/ml in SM. The same protocol was used for sporulating G1-arrested cells except that cells were pregrown to a higher density as described (Oelschlaegel et al. 2005). The ectopic expression of SMK1-HA in mitotic cells was achieved with the 2μ-based pSL6 plasmid that contains a mutant promoter lacking the Sum1 repressor binding site (Schaber et al. 2002).
Figure 1.
Activation of Smk1 in sporulation-defective deletion strains. (A) Meiotic kinetics in the parent strain used for constructing the panel of deletion strains containing or lacking a multicopy IME1 plasmid as indicated. (B) Strains cotransformed with a plasmid containing SMK1-HA and a plasmid overproducing IME1 were analyzed by Western immunoblot assay 24 hr after transferring cells to sporulation medium. The slower-migrating immunoreactive species corresponds to Smk1 that is phosphorylated in its activation loop (P-Smk1). The faster-migrating immunoreactive species corresponds to Smk1 that is not phosphorylated (Smk1). A cross-reacting species (*) is seen in mitotic and meiotic cells lacking the SMK1-HA plasmid and was used as a loading control.
TABLE 1.
Yeast Strains
| Strain | Genotype | Source |
|---|---|---|
| LNY150 | MATa/MATα ura3/ura3 leu2∷hisG/leu2∷hisG trp1∷hisG/trp1∷hisG his4-N/his4-G lys2/lys2 ho∷LYS2/ho∷LYS2 | Lenore Neigeborne |
| ALY60 | MATa/MATα SMK1-HA∷kan/SMK1-HA∷kan ura3/ura3 leu2∷hisG/leu2∷hisG trp1∷hisG/trp1∷hisG his4/his4 lys2/lys2 ho∷LYS2/ho∷LYS2 | This study |
| CMY15 | ALY60 + ama1∷kanMX6/ama1∷kanMX6 | This study |
| CMY24 | ALY60 + spo13∷hisG/spo13∷hisG | This study |
| CMY23 | ALY60 + spo13∷hisG/spo13∷hisG ama1∷kanMX6/ama1∷kanMX6 | This study |
| CMY25 | ALY60 + spo11∷hisG-URA3-hisG/spo11∷hisG-URA3-hisG | This study |
| CMY20 | ALY60 + spo11∷hisG-URA3-hisG/spo11∷hisG-URA3-hisG ama1∷kanMX6/ama1∷kanMX6 | This study |
| CMY21 | ALY60 + spo13∷hisG/spo13∷hisG spo11∷hisG-URA3-hisG/spo11∷hisG-URA3-hisG | This study |
| CMY22 | ALY60 + spo13∷hisG/spo13∷hisG spo11∷hisG-URA3-hisG/spo11∷hisG-URA3-hisG ama1∷kanMX6/ama1∷kanMX6 | This study |
| CMY48 | ALY60 + cdc28-4/cdc28-4 | This study |
| CMY37 | ALY60 + cd28-4/cdc28-4 ama1∷kanMX6/ama1∷kanMX6 | This study |
| CMY30 | MATa/MATα cdc14∷kanMX6/cdc14∷kanMX6 leu2∷cdc14-1∷LEU2∷TRP1/leu2∷cdc14-1∷LEU2∷TRP1 SMK1-HA∷kan/SMK1-HA∷kan ho∷LYS2/ho∷LYS2 lys2/lys2 ura3/ura3 trp1/trp1 his4 or HIS4 | This study |
| CMY31 | MATa/MATα slk19∷kanMX6/slk19∷kanMX6 SMK1-HA∷kan/SMK1-HA∷kan ho∷LYS2/ho∷LYS2 lys2/lys2 trp1/trp1 ura3/ura3 his4 or HIS4 | This study |
| CMY32 | MATa/MATα spo12∷LEU2/spo12∷LEU2 SMK1-HA∷kan/SMK1-HA∷kan ho∷LYS2/ho∷LYS2 lys2/lys2 leu2/leu2 trp1/trp1 ura3/ura3 his4 or HIS4 | This study |
Biochemical and cellular assays:
Cellular extracts were prepared from aliquots of sporulating cells collected at different times postinduction by the NaOH lysis/trichloroacetic acid precipitation procedure (Yaffe and Schatz 1984) and analyzed for Smk1-HA as previously described (Schaber et al. 2002). Immunoblot analysis of histone H4 was carried out using rabbit antisera prepared against yeast histone H4 (Abcam). For determining viability of sporulated cells, asci (or cells) 48 hr postinduction were counted using a hemocytometer and plated on rich medium, and colony formation was scored. For determining glusulase resistance, 107 asci were washed twice in 0.1 m PIPES pH 6.8, resuspended in glusulase diluted 1:5 in the same buffer, and digested for 1.5 hr at 30°. After digestion, cells were washed twice in water, serial dilutions were plated on rich medium, and colony formation was scored. Fluorescence assays were carried out on cells that were sporulated in liquid medium. Cells were spotted drop-wise onto nitrocellulose filters using a fritted glass funnel under vacuum. This modification to the fluorescence plate assay was necessary since cdc28-4 mutants do not adhere to filters by simple replica plating. The filters were processed as previously described (Wagner et al. 1997).
RESULTS
An assay for Smk1 activation-defective mutants:
Enyenihi and Saunders (2003) previously assayed sporulation in the panel of viable homozygous deletion strains developed by the Saccharomyces Genome Deletion Project. We reasoned that a subset of the sporulation-defective mutants reported in this screen might be deficient in activating Smk1. One assay that can be used to monitor the activation state of Smk1 is electrophoretic mobility in SDS-polyacrylamide gels. In this assay, Smk1 from sporulating cells migrates as a doublet. The slower migrating form is phosphorylated in the T-X-Y motif (T207, Y209) that corresponds to the activation loops in other MAPKs (Schaber et al. 2002). Similar to other MAPKs, the maximal ratio of phosphorylated:unphosphorylated Smk1 is ∼1:1. The ratio of these electrophoretic forms provides a semiquantitative measure of Smk1's activation state. To identify activation-defective mutants, a plasmid encoding an epitope-tagged (HA) allele of SMK1 (pSL1) was used (Schaber et al. 2002). The genetic background used to construct the mutant bank (S288C) initiates meiosis asynchronously and slowly. Because of this, and because Smk1 is expressed only transiently during meiosis, Smk1-HA immunoreactivity in meiotic cultures assayed at different times postinduction was below the limit of detection. Overexpression of IME1 using a multicopy pS303 plasmid increased the percentage of cells in the S288C background that induced meiosis, the rate at which they transit through the program, and the overall synchrony (Figure 1A). We found that Smk1-HA was readily detected in meiotic cells harboring both the pSL1 Smk1-HA and the pS303 multicopy IME1 plasmids (Figure 1B).
Homozygous deletion diploids from the collection were cotransformed with the SMK1-HA and the multicopy IME1 plasmids, and the expression of Smk1-HA was tested in sporulating cultures. The strains that were screened in this study include the subset of mutants scored as sporulation defective in the Enyenihi and Saunders study that have also been reported to complete meiosis (a smk1-like phenotype). In addition, a number of mutants that have characterized defects prior to spore formation were screened to gain insight into dependency relationships that influence Smk1 expression or its activation state. We also included certain mutants in this analysis on the basis of whether a gene is expressed during meiosis, sequence data, and other criteria from the published literature. A total of 86 homozygous single-gene deletion mutants were tested using this approach. This screen led to the sorting of mutants into three phenotypic classes with respect to Smk1 expression/activation (Table 2). A representative example of the data is shown in Figure 1B. Class I mutants did not express detectable levels of Smk1-HA in our assay (30 members). Class II mutants expressed Smk1-HA to detectable levels and the ratio of active:inactive kinase was indistinguishable from wild type (54 members). Class III mutants expressed Smk1 and had a low relative amount of activated Smk1 (2 members). We did not identify any mutants in which there was a higher-than-normal relative amount of activated Smk1. The three classes of mutants are described, in turn, below.
Class I mutants:
Smk1-HA was undetectable in at least three independent cotransformants for each of the 30 class I mutant strains in Table 2. All of the class I mutants failed to complete meiosis as assayed by DAPI staining. The most likely interpretation of these results is that class I mutants block in the program prior to the stage in the program when Smk1 is expressed.
Most middle promoters rely heavily on the transcriptional activation function of Ndt80 and are expressed very poorly in its absence. However, previous studies have shown that the SMK1 middle promoter is somewhat atypical in this respect since removal of Sum1 is sufficient to induce its expression to moderate levels even when Ndt80 is not expressed (Xie et al. 1999; Lindgren et al. 2000). A ndt80 mutant produces Smk1 protein levels that are only a fewfold lower than that seen in the wild type (Figure 1B), which is consistent with these previous results. Thus, the failure to express Smk1 in this study suggests that a given mutant has failed to remove Sum1 repression (see discussion). A caveat to interpreting the data in this fashion is that the class I mutants in which multicopy IME1 can no longer promote meiosis, including those lacking the IME1 pathway genes IME2 and RIM11, are not informative since the multicopy IME1 plasmid is required for Smk1 detection even in the wild-type background.
Class II mutants:
Class II mutants expressed Smk1-HA and the ratio of phosphorylated:unphosphorylated kinase was indistinguishable from that of wild type. Mutants defective in processing recombination intermediates (dmc1), in synaptonemal complex formation (zip1), exit from pachytene (ndt80), exit from meiosis I (slk19, spo12), prospore membrane formation (spo20, spo21, mpc54), deposition of all spore wall layers (gip1), and assembly of specific spore wall layers (spo73, spo77) accumulated Smk1 that was maximally phosphorylated. Thus, once Smk1 is translated, the activation of Smk1 by phosphorylation does not appear to be controlled through a simple dependency relationship by any of these steps in the sporulation program.
Class III mutants:
Class III consists of only two members. These mutants are in AMA1, which encodes a meiosis-specific Cdc20 family member (Cooper et al. 2000), and in SSP2, which encodes a protein of unknown biochemical function that has previously been shown to be required for spore wall formation (Sarkar et al. 2002). Here, we describe our analysis of AMA1. Our analysis of SSP2 will be presented elsewhere.
Ama1 is required for Smk1 activation during meiosis:
The SK1 strain background induces sporulation efficiently and transits through the program relatively quickly. We have previously characterized the expression of Smk1 and its activation in this background (Krisak et al. 1994; Pierce et al. 1998; Lindgren et al. 2000; Schaber et al. 2002). In these studies, Smk1 protein was produced in wild-type cells starting at ∼5 hr and accumulated to maximal levels by 10 hr postinduction. During this interval, >90% of cells completed MI and MII. As the meiotic divisions are completed, and Smk1 levels increase, its phosphorylation state increases (Schaber et al. 2002). To further investigate the role of AMA1 in regulating Smk1, the Smk1-HA protein was monitored in a series of mutant SK1 strains at different times after transfer to sporulation medium (Figure 2). Smk1 accumulated with similar kinetics in the wild-type and ama1 mutant. However, the Smk1 that was produced in the ama1 mutant was predominantly of the nonphosphorylated form, consistent with the results obtained in the S288C background. When Smk1 is first produced, a similar low relative amount of phosphorylated Smk1 was seen in both the ama1 and wild-type strains. However, as meiosis proceeded in the ama1 strain and total Smk1 levels increased, only the nonphosphorylated form accumulated so that at later time points <10% of the Smk1 present was activated by phosphorylation. In contrast, ∼50% of the Smk1 was activated in the wild-type strain at these time points. These results confirm the screen results and show that ama1 mutants are defective in Smk1 activation.
Figure 2.
AMA1 is required for full activation of Smk1 during sporulation. SK1 diploids containing an insertion of an HA-epitope-coding region at the carboxy-terminal coding region of SMK1 and lacking the indicated genes were incubated in sporulation medium. Extracts prepared from cells isolated at the indicated times were analyzed by Western immunoblot analysis using an HA antibody (Smk1-HA). The wild-type and ama1 samples were also analyzed by immunoblot analysis using an antiserum directed against histone H4 as indicated.
The role of Ama1 in Smk1 activation is independent of MI and/or genetic recombination:
Strains lacking SPO13 can skip MI and complete MII to form dyad spores after a delay. In the spo13 strain, Smk1 is expressed but its accumulation is delayed modestly (Figure 2). Once expressed, the Smk1 in a spo13 mutant is activated by phosphorylation as seen in wild-type cells. In contrast, the ama1 spo13 mutant shows an Smk1 activation defect. Mutants lacking SPO11 do not initiate recombination and transit through the program to form inviable spores (Keeney et al. 1997). We compared Smk1 in the spo13 spo11 background containing or lacking Ama1. As a control, Smk1 in the spo11 background was also tested. Removal of SPO11 advanced the kinetics of Smk1 expression in both the wild-type and spo13 backgrounds, consistent with the meiotic advance reported for spo11 single mutants (Kee and Keeney 2002; Malone et al. 2004). Removal of AMA1 delayed the kinetics of Smk1 expression modestly in all of the genetic backgrounds tested. The kinetic delays are consistent with a role for Ama1 in promoting meiosis-specific events but not being required for their completion (Oelschlaegel et al. 2005). Smk1 is still not activated at late time points in the ama1 spo11 or in the ama1 spo13 spo11 backgrounds. Thus, the function of Ama1 in regulating Smk1 activation is independent of genetic recombination or MI.
It is notable that Smk1 is present in the 24-hr ama1 extracts but absent from the 24-hr wild-type extracts. By 24 hr, wild-type SK1 diploids complete spore wall formation and maturation. In contrast, ama1 cells are blocked prior to deposition of spore wall layers (see below). To test whether protein found in the spore interior is efficiently extracted using the conditions tested, control immunoblot analyses were carried out using histone H4 antisera. H4 is extracted in an apparently quantitative manner from all samples tested except from the wild-type 24-hr spore sample (Figure 2, bottom). We conclude that the presence of Smk1-HA in extracts prepared from ama1 but not wild-type cells at late time points could reflect poor or selective extraction of protein from wild-type spores using the method tested.
Mutations in CDC28 can suppress the sporulation defect of an ama1 mutant:
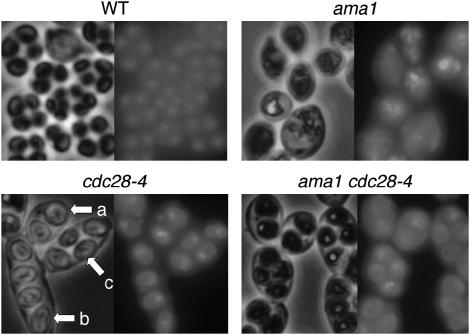
Smk1 activation occurs as cells are completing the meiotic divisions and begining spore wall formation. Downregulation of CDK is required to exit from the meiotic and mitotic divisions. To investigate whether Ama1 could be exerting its effect on Smk1 in a pathway with the Cdc28 CDK, we used the temperature-sensitive cdc28-4 allele, which in the SK1 genetic background supports mitotic growth at 30° but not at 34°. We tested the meiosis and sporulation phenotypes of cdc28-4, ama1, and ama1 cdc28-4 mutants by microscopy of DAPI-stained cultures (Figure 3). At 30°, cdc28-4 diploids completed meiosis and formed spores although they did so at a modestly reduced frequency (both meiosis and spore formation occur at 60–65% compared to >90% completion of both processes in the wild-type cultures). The asci formed in the cdc28-4 background often appear elongated, containing spores in a linear instead of tetrahedral array, or are otherwise misshapen (Figure 3, bottom left). This is likely a consequence of the increased percentage of mitotic cells that are elongated in the cdc28-4 mutant since the shape of the ascal wall is influenced by the shape of the vegetative wall from which it is derived. In addition, a high percentage of cdc28-4 cells formed dyads, consistent with a previous study showing that cdc28-4 cells sporulated at a semipermissive temperature often skip MII and form dyad spores (Shuster and Byers 1989). In the ama1 mutant, nuclei are often fragmented (Figure 3, top right) and we have never observed spores. These results are consistent with previous studies that have documented an early and apparently complete block in the spore wall morphogenesis pathway in ama1 mutants (Cooper et al. 2000; Coluccio et al. 2004). Remarkably, most of the ama1 cdc28-4 cells form spores (Figure 3, bottom right).
Figure 3.
The cdc28-4 mutation can suppress sporulation defects of ama1 strains. Cells of the indicated genotype were incubated in sporulation medium for 24 hr, stained with the DNA intercalator DAPI, and photographed under visible light (left) and UV/fluorescence (right). The arrows in the cdc28-4 panel show dyad spores (a), elongated spores (b), and tetrads arranged in a single plane (c), which are all found at increased frequencies in the cdc28-4 background.
Dityrosine is a UV-absorbing component of the outer spore wall and fluorescence can be used to monitor its accumulation. Wild-type and cdc28-4 diploids sporulated at 30° fluoresced indistinguishably in this assay (Figure 4A). The ama1 diploid was negative in the fluorescence assay. The cdc28-4 mutation suppressed the fluorescence defect of the ama1 strain consistent with the microscopy.
Figure 4.
cdc28-4 can suppress the fluorescence, viability, and enzymatic resistance defects of ama1 strains. (A) Fluoresence assays for sporulation were performed on three isolates of the indicated strains. (B) Homozygous diploids of the indicated genotype that had been incubated in sporulation medium for 48 hr were counted using a hemocytometer and plated on rich medium, and colonies formed after 3 days growth counted to score viability. (C) As in B except that cells were digested with glusulase for 1.5 hr and washed in water prior to plating. Data in both B and C are expressed relative to wild type, which was set at 100% (see text). The standard deviation for each strain tested in B and C is <22% and represents an average of four different isolates of the indicated genotype derived from two independently generated cdc28-4 and ama1 mutants.
To further characterize the interactions between the cdc28-4 and ama1 mutations, we measured the viability of the meiotic products formed in ama1, cdc28-4, and ama1 cdc28-4 strains before and after digestion with glusulase (Figure 4, B and C, respectively). All of the asci formed by the wild-type strain were able to form colonies when directly plated on rich medium (1.14 ± 0.3 CFU/ascus) and the spores formed were mostly resistant to glusulase digestion (3.2 ± 0.5 CFU/ascus). In contrast to the wild-type strain, the viability of the ama1 mutant before glusulase digestion was dramatically reduced (1 × 10−4 CFU/meiotic cell; Figure 4B). This result is consistent with the nuclear fragmentation phenotype of meiotic ama1 cells (Figure 3). Digestion with glusulase decreased the number of ama1 colonies further (Figure 4C). Interestingly, most (98%) of the cdc28-4 sporulated cells were also inviable despite the fact that many (60–65%) form spores as assayed microscopically. Digestion with glusulase only modestly reduced the number of colonies formed, indicating that most viable cdc28-4 asci contained glusulase-resistant spores. Remarkably, the viability of asci formed by the ama1 cdc28-4 double-mutant strain is almost as high as the viability of asci formed by wild-type cells (Figure 4B). In addition, the spores formed by the ama1 cdc28-4 strain were as resistant to glusulase as wild type (Figure 4C). These data show that the cdc28-4 mutation can suppress meiosis-induced inviability defects caused by the ama1 mutation and vice versa. In addition, these data show that the cdc28-4 mutation can suppress the glusulase resistance defect of an ama1 strain. Resistance to glusulase digestion requires a higher level of Smk1 activity than does formation of spores as assayed microscopically or by using the fluorescence assay (Wagner et al. 1999) and is a relatively stringent criterion for scoring formation of mature spores. Thus these data show that the cdc28-4 mutation suppresses the spore formation defect of an ama1 strain using the most stringent assay tested.
Sporulated ama1 cells contained supernumerary DAPI-staining foci that often appeared diffuse, fragmented, and irregularly arranged (Figure 3; top right). These observations raised the possibility that DNA masses were being fragmented in ama1 cells during or shortly after chromosome segregation. In contrast, a recent report by Oelschlaegel et al. (2005) showed that ama1 mutants completed meiosis indistinguishably from wild-type cells (but failed to form spores). In the Oelschlaegel study, cells were grown to a high density prior to transferring them to sporulation medium, resulting in the accumulation of cells in G1 (referred to as “G1-arrested” below) whereas our study started with logarithmically growing cells. We found that the nuclear-fragmentation phenotype was less severe and that cells were mostly viable when G1-arrested ama1 cells were used compared to dividing cells (4 × 10−1 CFU/cell for G1-arrested compared to 1 × 10−4 CFU/cell for the dividing cell culture). We speculate that the lethality of ama1 sporulated cells is a consequence of nuclear fragmentation and that a previously unrecognized pathway is induced in G1-starved cells that can suppress this fragmentation phenotype. Carrying out the experiments in different concentrations of acetate does not suppress DNA fragmentation or lethality, suggesting that these are not simple osmotic-remedial phenotypes. Suppression of the meiosis-induced ama1 lethality by cdc28-4 could in part be a secondary consequence of a G1 arrest-like (START) phenotype. However, it is not possible to explain the fluorescence, microscopic, and enzymatic resistance data in this manner. While the basis of the inviability of the cdc28-4 spores remains unclear, its suppression by removal of AMA1 indicates that Ama1 can influence Cdc28's activity state during meiosis. In total, the suppression data support a model in which Ama1 controls Cdc28, which in turn controls Smk1.
Smk1 activation was next compared in the ama1, cdc28-4, and the ama1 cdc28-4 strains. The Smk1 activation defect of an ama1 strain is partially suppressed by the cdc28-4 mutation (Figure 5A). While the increase in the relative amount of activated Smk1 in the ama1 cdc28-4 strain compared to the ama1 strain is reproducible between experiments (n = 3) and seen at all time points tested, the magnitude of the increase is modest. To further characterize the regulation of Smk1 by Cdc28, the activation state of Smk1-HA was monitored in wild-type and cdc28-4 mitotic cells. For these experiments a mutant SMK1 promoter lacking the Sum1 binding site (MSEs) was used to derepress the SMK1 promoter in mitotic haploid cells (Schaber et al. 2002). As shown in Figure 5B and as previously reported (Schaber et al. 2002), Smk1 is activated to a lower level in mitosis than in meiosis. The phosphorylation state of ectopically expressed Smk1 was increased in the cdc28-4 mitotic haploid (but not to the maximally phosphorylated level seen in meiotic cells). These data further support the conclusion that Cdc28 negatively regulates Smk1 and demonstrates that at least some of this regulation can occur in mitotic cells where Ama1 is absent.
Figure 5.
The cdc28-4 mutation suppresses the Smk1 activation defect of ama1 cells in meiosis and increases the fraction of Smk1 that is phosphorylated in mitosis. (A) Assays of Smk1-HA in the indicated strains were performed as described in Figure 2. (B) Smk1-HA was expressed in mitotic cells of the indicated genotypes using a mutant SMK1 promoter lacking the Sum1-dependent repression site (pSL-6), and Smk1-HA protein was assayed by immunoblot analysis.
The FEAR/Cdc14 and the Ama1/Cdc28/Smk1 pathways function independently:
It has previously been shown that exit from mitosis is controlled by the FEAR/MEN network that mediates the release of the Cdc14 phosphatase from the nucleolus. Cdc14 acts, at least in part, by reversing phospho-modifications that are catalyzed by the Cdc28 protein kinase. The FEAR network also regulates exit from meiosis I through Cdc14 and cells that are deficient in Cdc14 activity fail to disassemble the MI spindle (Buonomo et al. 2003; Marston et al. 2003). Spo12 and Slk19 are components of the FEAR network and cells lacking these proteins fail to disassemble the MI spindle in a timely fashion, leading to two rounds of chromosome segregation on a MI spindle. Despite the persistent MI spindle in the spo12 or slk19 strains, these cells do eventually form spores to generate dyads. Cells homozygous for the cdc14-1 temperature-sensitive mutation also undergo two rounds of chromosome segregation on a persistent MI spindle. At the nonpermissive temperature, however, the spindle is rarely disassembled and these cells fail to form spores. Our findings that Ama1 regulates Smk1 in a Cdc28-dependent fashion and that the FEAR network acts in opposition to Cdc28 raised the possibility that this regulatory network could control Smk1 activation. To test this possibility, we examined Smk1 during meiosis in spo12 or slk19 strains. We also examined Smk1 in a cdc14-1 temperature-sensitive strain. Smk1 expression and activation were unaffected in the spo12 and the slk19 diploids (Figure 6). This result is consistent with the screen results showing that spo12 and the slk19 diploids did not effect Smk1 activation in the S288C genetic background (Table 2) and with the observation that these strains form spores (Buonomo et al. 2003; Marston et al. 2003). The cdc14-1 strain also expressed and activated Smk1 indistinguishably from wild type at 30° where chromosomes are segregated on the MI spindle but where dyad spores are not formed (Figure 6). These results suggest that although SPO12, SLK19, and CDC14 act in opposition to CDC28 to regulate exit from MI, this regulatory network does not control Smk1's activation state.
Figure 6.
Smk1 expression/activation is not controlled via the FEAR network. Smk1 was assayed in meiotic cells of the indicated genotypes as described in Figure 2.
DISCUSSION
In this study, a subset of the homozygous single-gene deletion diploids have been tested using a direct biochemical assay to identify mutants defective in Smk1 regulation. These mutants show defects in two different pathways that control Smk1. The first pathway controls whether Smk1 is expressed while the second pathway controls whether Smk1 is activated by phosphorylation once it is produced.
Class I mutants and the control of Smk1 expression:
In previous studies it was shown that the competition of Sum1 and Ndt80 for MSEs in middle-gene promoters controls middle-gene induction (Xie et al. 1999; Pierce et al. 2003). The SMK1 promoter contains a MSE that binds Sum1 with high affinity (Pierce et al. 1998), and SMK1 is expressed when Sum1 repression has been removed, even in the absence of Ndt80 (Lindgren et al. 2000; Pak and Segall 2002b). Defining the steps in sporulation that that must be completed to permit Smk1 expression is important not only for understanding the Smk1 pathway but also for our broader understanding of meiotic regulation since Sum1 controls exit from pachytene and entry into the meiotic divisions (Lindgren et al. 2000; Pak and Segall 2002b).
In this study we have shown that mutants defective in key steps in sporulation, including premeiotic DNA replication (mum2), early steps in homolog pairing (hop2, rec8), and double-strand-break (DSB) site selection/formation (rad6, bre1), failed to express Smk1. Notably, mutants defective in synapsis (zip1) and in processing DSBs into recombination intermediates (dmc1) did express Smk1 in our screen. The spo11 mutant, which fails to initiate recombination, expressed wild-type levels of Smk1, consistent with the ability of spo11 strains to form spores (Keeney et al. 1997). Thus, it appears that once recombination has been initiated that some but not all steps in the pathway must be completed to permit Smk1 derepression. Interestingly, mutants lacking the Spo1 phospholipase-B homolog failed to express Smk1 (Table 2). A spo1 mutant completes S phase and genetic recombination but stalls in prophase with unduplicated spindle pole bodies (SPBs) (Tevzadze et al. 2000). ndt80 mutants express Smk1 (Figure 1) and block in pachytene with duplicated but unseparated SPBs (Xu et al. 1995). These data suggest that Sum1's ability to repress middle-gene promoters may be connected to a Spo1-dependent step that occurs at or just prior to SPB duplication.
Class II mutants and the control of Smk1 activation:
Many mutants that show defects in prophase as well as a variety of well-characterized steps in prospore membrane assembly or spore wall formation accumulate Smk1 that has been activated by phosphorylation. Thus, blocking these key steps in sporulation does not prevent Smk1 activation. The fact that Smk1 is phosphorylated normally in mutants that block prior to meiosis and in mutants that block in the postmeiotic steps of prospore membrane or spore wall formation suggest that the Cdc28-dependent repression pathway that limits Smk1 phosphorylation is not enforced as these steps are occurring.
Class III mutants and a pathway that prevents Smk1 activation during meiotic chromosome segregation:
We assayed all of the viable sporulation-defective mutants described in the Enyenihi and Saunders (2003) study that have also been reported to complete meiosis. Of this set of mutants, only the ssp2 and the ama1 mutants showed demonstrable Smk1 activation defects. Thus, it appears that only a few nonessential genes are exclusively required for Smk1 to become activated by phosphorylation.
In previous studies we showed that the essential CDK-activating kinase Cak1 is required to activate Smk1 (Wagner et al. 1997). This activity is separable from its role in phosphorylating Cdc28, which also takes place during meiosis (Schaber et al. 2002; Schindler et al. 2003). This dual function of Cak1 in meiosis is seemingly paradoxical since in this study we have shown that Cdc28 can inhibit Smk1 activation. However, in wild-type cells, the regulatory role of Cak1 in the Smk1 pathway appears to be exclusively positive since the Cak1/Cdc28 reaction is constitutive and does not appear to be rate limiting for Cdc28 function (Kaldis et al. 1998; Schindler et al. 2003). ssp2 mutants complete the meiotic divisions on schedule and block in spore wall formation (Sarkar et al. 2002). Our results suggest that Ssp2 functions to promote Smk1 phosphorylation. One possibility is that Ssp2 promotes a functional signaling interaction between Cak1 and Smk1. Further experiments will be required to elucidate the role of Ssp2 in the Smk1 activation pathway.
The major findings of this study are: (1) the Ama1 activator of the APC/C promotes Smk1 activation, (2) Cdc28 inhibits Smk1 activation, and (3) mutations in CDC28 suppress the Smk1 activation defect and the sporulation defect caused by deletion of AMA1. These data support a model in which Ama1 controls Smk1 through Cdc28. One possibility is that Cdc28 inhibits a step (or steps) required for Smk1 activation and that Ama1 is required to relieve this inhibition by downregulating Cdc28. At this point, it remains possible that Ama1 also has a second function downstream of Cdc28 in promoting Smk1 activation (for example, by targeting a Cdc28-phosphorylated inhibitor of Smk1 for APC/C-mediated ubiquitylation).
It has recently been shown that Ama1 activity is inhibited by the APC/C subunit Mnd2 (Oelschlaegel et al. 2005; Penkner et al. 2005). Mnd2 disappears as cells complete MII. The increase in Ama1's activity that accompanies Mnd2's destruction could be a key regulatory step that controls Smk1 activation, thus coupling activation of the Smk1 MAPK to completion of meiosis. In the Oelschlaegel et al. study it was shown that Cdc28 regulates Ama1 activity. In contrast, our genetic suppression data (Figures 3 and 4) indicate that Ama1 regulates Cdc28. The ability of Cdc28 to inhibit Smk1 activation in mitotic cells (Figure 5B) shows that Cdc28 can regulate Smk1 even when Ama1 is absent. Taken together, the available data suggest that Ama1 regulates Cdc28 (this study) and that it is also regulated by Cdc28 (Oelschlaegel et al. 2005). The data suggest that Cdc28, Ama1, and Mnd2 contribute to a self-reinforcing regulatory loop to promote an irreversible and switch-like decrease in Clb/Cdc28 activity. One consequence of this decrease in Clb/Cdc28 is removal of Smk1 inhibition, thus coupling spore formation to exit from MII. It is unclear why deletion of AMA1 has little effect on the meiotic divisions yet is absolutely required for spore formation. In this regard it is interesting that mutations in FEAR network components and CDC14 that control exit from MI through Cdc28 do not appear to affect Smk1 activation. One possibility is that the pool of Cdc28 that regulates Smk1 is distinct (either spatially or otherwise) from the pool of Cdc28 that regulates the meiotic divisions.
In summary, this report shows that Cdc28 negatively regulates the Smk1 sporulation-specific MAPK that controls the postmeiotic program of spore morphogenesis and that the meiosis-specific Ama1 APC-targeting subunit is required to relieve this inhibition. The combined activities of these proteins form an integrated regulatory pathway that couples gametogenesis to the completion of meiosis. The presence of meiosis-specific Cdc20 homologs required for gamete formation in evolutionarily diverse organisms makes it likely that components of the Ama1/Cdc28/Smk1 pathway are shared in germ cell development in other organisms. We speculate that features of this pathway may also play a role in linking the completion of mitosis to morphogenetic programs during differentiation of somatic cells.
Acknowledgments
We thank Angelika Amon, Nancy Kleckner, Adele Marston, and Lenore Neigeborn for yeast strains and Steven Kron for plasmids. We also thank William Saunders for yeast strains and advice about sporulating the Saccharomyces deletion collection. We are also indebted to Karen Schindler, Erica Johnson, and Randy Strich for helpful discussions and comments on this manuscript. This work was supported by National Institutes of Health grant GM061817 (to E.W.) and American Cancer Society grant CCG106162 (to K.F.C.).
References
- Asakawa, H., K. Kitamura and C. Shimoda, 2001. A novel Cdc20-related WD-repeat protein, Fzr1, is required for spore formation in Schizosaccharomyces pombe. Mol. Genet. Genomics 265: 424–435. [DOI] [PubMed] [Google Scholar]
- Benjamin, K. R., C. Zhang, K. M. Shokat and I. Herskowitz, 2003. Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev. 17: 1524–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco, M. A., L. Pelloquin and S. Moreno, 2001. Fission yeast mfr1 activates APC and coordinates meiotic nuclear division with sporulation. J. Cell Sci. 114: 2135–2143. [DOI] [PubMed] [Google Scholar]
- Buonomo, S. B., K. P. Rabitsch, J. Fuchs, S. Gruber, M. Sullivan et al., 2003. Division of the nucleolus and its release of CDC14 during anaphase of meiosis I depends on separase, SPO12, and SLK19. Dev. Cell 4: 727–739. [DOI] [PubMed] [Google Scholar]
- Chu, S., and I. Herskowitz, 1998. Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol. Cell 1: 685–696. [DOI] [PubMed] [Google Scholar]
- Chu, S., J. DeRisi, M. Eisen, J. Mulholland, D. Botstein et al., 1998. The transcriptional program of sporulation in budding yeast. Science 282: 699–705 (erratum: Science 282: 1421). [DOI] [PubMed] [Google Scholar]
- Coluccio, A., E. Bogengruber, M. N. Conrad, M. E. Dresser, P. Briza et al., 2004. Morphogenetic pathway of spore wall assembly in Saccharomyces cerevisiae. Eukaryot. Cell 3: 1464–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, K. F., M. J. Mallory, D. B. Egeland, M. Jarnik and R. Strich, 2000. Ama1p is a meiosis-specific regulator of the anaphase promoting complex/cyclosome in yeast. Proc. Natl. Acad. Sci. USA 97: 14548–14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amours, D., and A. Amon, 2004. At the interface between signaling and executing anaphase–Cdc14 and the FEAR network. Genes Dev. 18: 2581–2595. [DOI] [PubMed] [Google Scholar]
- Enyenihi, A. H., and W. S. Saunders, 2003. Large-scale functional genomic analysis of sporulation and meiosis in Saccharomyces cerevisiae. Genetics 163: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, J. W., J. L. Burton and M. J. Solomon, 2002. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 16: 2179–2206. [DOI] [PubMed] [Google Scholar]
- Honigberg, S. M., and R. H. Lee, 1998. Snf1 kinase connects nutritional pathways controlling meiosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 18: 4548–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg, S. M., and K. Purnapatre, 2003. Signal pathway integration in the switch from the mitotic cell cycle to meiosis in yeast. J. Cell Sci. 116: 2137–2147. [DOI] [PubMed] [Google Scholar]
- Kaldis, P., Z. W. Pitluk, I. A. Bany, D. A. Enke, M. Wagner et al., 1998. Localization and regulation of the cdk-activating kinase (Cak1p) from budding yeast. J. Cell Sci. 111: 3585–3596. [DOI] [PubMed] [Google Scholar]
- Kassir, Y., D. Granot and G. Simchen, 1988. IME1, a positive regulator gene of meiosis in S. cerevisiae. Cell 52: 853–862. [DOI] [PubMed] [Google Scholar]
- Kee, K., and S. Keeney, 2002. Functional interactions between SPO11 and REC102 during initiation of meiotic recombination in Saccharomyces cerevisiae. Genetics 160: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney, S., C. N. Giroux and N. Kleckner, 1997. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88: 375–384. [DOI] [PubMed] [Google Scholar]
- Krisak, L., R. Strich, R. S. Winters, J. P. Hall, M. J. Mallory et al., 1994. SMK1, a developmentally regulated MAP kinase, is required for spore wall assembly in Saccharomyces cerevisiae. Genes Dev. 8: 2151–2161. [DOI] [PubMed] [Google Scholar]
- Lindgren, A., D. Bungard, M. Pierce, J. Xie, A. Vershon et al., 2000. The pachytene checkpoint in Saccharomyces cerevisiae requires the Sum1 transcriptional repressor. EMBO J. 19: 6489–6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M. S., A. McKenzie, III, D. J. Demarini, N. G. Shah, A. Wach et al., 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Lorincz, A. T., and S. I. Reed, 1986. Sequence analysis of temperature-sensitive mutations in the Saccharomyces cerevisiae gene CDC28. Mol. Cell. Biol. 6: 4099–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone, R. E., S. J. Haring, K. E. Foreman, M. L. Pansegrau, S. M. Smith et al., 2004. The signal from the initiation of meiotic recombination to the first division of meiosis. Eukaryot. Cell 3: 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston, A. L., B. H. Lee and A. Amon, 2003. The Cdc14 phosphatase and the FEAR network control meiotic spindle disassembly and chromosome segregation. Dev. Cell 4: 711–726. [DOI] [PubMed] [Google Scholar]
- Mendenhall, M. D., and A. E. Hodge, 1998. Regulation of Cdc28 cyclin-dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62: 1191–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelschlaegel, T., M. Schwickart, J. Matos, A. Bogdanova, A. Camasses et al., 2005. The yeast APC/C subunit Mnd2 prevents premature sister chromatid separation triggered by the meiosis-specific APC/C-Ama1. Cell 120: 773–788. [DOI] [PubMed] [Google Scholar]
- Pak, J., and J. Segall, 2002. a Regulation of the premiddle and middle phases of expression of the NDT80 gene during sporulation of Saccharomyces cerevisiae. Mol. Cell. Biol. 22: 6417–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak, J., and J. Segall, 2002. b Role of Ndt80, Sum1, and Swe1 as targets of the meiotic recombination checkpoint that control exit from pachytene and spore formation in Saccharomyces cerevisiae. Mol. Cell. Biol. 22: 6430–6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkner, A. M., S. Prinz, S. Ferscha and F. Klein, 2005. Mnd2, an essential antagonist of the anaphase-promoting complex during meiotic prophase. Cell 120: 789–801. [DOI] [PubMed] [Google Scholar]
- Pierce, M., M. Wagner, J. Xie, V. Gailus-Durner, J. Six et al., 1998. Transcriptional regulation of the SMK1 mitogen-activated protein kinase gene during meiotic development in Saccharomyces cerevisiae. Mol. Cell. Biol. 18: 5970–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, M., K. R. Benjamin, S. P. Montano, M. M. Georgiadis, E. Winter et al., 2003. Sum1 and Ndt80 proteins compete for binding to middle sporulation element sequences that control meiotic gene expression. Mol. Cell. Biol. 23: 4814–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primig, M., R. Williams, E. Winzeler, G. Tevzadze, A. Conway et al., 2000. The core meiotic transcriptome in budding yeasts. Nat. Genet. 26: 415–423. [DOI] [PubMed] [Google Scholar]
- Rabitsch, K., A. Toth, M. Galova, A. Schleiffer, G. Schaffner et al., 2001. A screen for genes required for meiosis and spore formation based on whole-genome expression. Curr. Biol. 11: 1001–1009. [DOI] [PubMed] [Google Scholar]
- Sarkar, P. K., M. A. Florczyk, K. A. McDonough and D. K. Nag, 2002. SSP2, a sporulation-specific gene necessary for outer spore wall assembly in the yeast Saccharomyces cerevisiae. Mol. Genet. Genomics 267: 348–358. [DOI] [PubMed] [Google Scholar]
- Schaber, M., A. Lindgren, K. Schindler, D. Bungard, P. Kaldis et al., 2002. CAK1 promotes meiosis and spore formation in Saccharomyces cerevisiae in a CDC28-independent fashion. Mol. Cell. Biol. 22: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, K., K. R. Benjamin, A. Martin, A. Boglioli, I. Herskowitz et al., 2003. The Cdk-activating kinase Cak1p promotes meiotic S phase through Ime2p. Mol. Cell. Biol. 23: 8718–8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster, E. O., and B. Byers, 1989. Pachytene arrest and other meiotic effects of the start mutations in Saccharomyces cerevisiae. Genetics 123: 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevzadze, G. G., H. Swift and R. E. Esposito, 2000. Spo1, a phospholipase B homolog, is required for spindle pole body duplication during meiosis in Saccharomyces cerevisiae. Chromosoma 109: 72–85. [DOI] [PubMed] [Google Scholar]
- Wagner, M., M. Pierce and E. Winter, 1997. The CDK-activating kinase CAK1 can dosage suppress sporulation defects of smk1 MAP kinase mutants and is required for spore wall morphogenesis in Saccharomyces cerevisiae. EMBO J. 16: 1305–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, M., P. Briza, M. Pierce and E. Winter, 1999. Distinct steps in yeast spore morphogenesis require distinct SMK1 MAP kinase thresholds. Genetics 151: 1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann, C., S. Gibson, M. B. Jarpe and G. L. Johnson, 1999. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79: 143–180. [DOI] [PubMed] [Google Scholar]
- Xie, J., M. Pierce, V. Gailus-Durner, M. Wagner, E. Winter et al., 1999. Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in Saccharomyces cerevisiae. EMBO J. 18: 6448–6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L., M. Ajimura, R. Padmore, C. Klein and N. Kleckner, 1995. NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol. Cell. Biol. 15: 6572–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe, M. P., and G. Schatz, 1984. Two nuclear mutations that block mitochondrial protein import in yeast. Proc. Natl. Acad. Sci. USA 81: 4819–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]