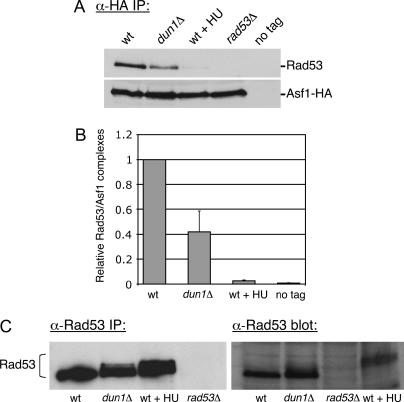
Figure 9.
Asf1/Rad53 complexes are less abundant in dun1Δ mutant cells. (A) Immunoblot analysis of Asf1-HA immunoprecipitates. Cell extracts prepared from ASF1-HA (PKY2735), dun1Δ ASF1-HA (PKY3607), ASF1-HA + 0.2 m HU (PKY2735), rad53Δ sml1Δ ASF1-HA (PKY2747), and ASF1 (PKY090) strains were subjected to immunoprecipitation with an anti-HA antibody. Eluates were probed with Rad53 and HA antibodies to compare relative recovery of Asf1-HA/Rad53 complexes. (B) Quantitation of Rad53 coprecipitation with Asf1-HA from wild-type and dun1Δ cell extracts. The efficiency of Rad53 coprecipitation with Asf1-HA was measured from three independent experiments using Quantity One software (Bio-Rad). In each experiment, recovery of Rad53 from the wild-type extract was normalized to 1.0. Average recovery of Rad53 and the standard deviation for each genotype are plotted on the graph. (C) Immunoblot analysis of phosphorylated Rad53: (Left) Cell extracts from the same strains as in A were subjected to immunoprecipitation with an anti-Rad53 antibody. Eluates were analyzed on a 7.5% SDS-PAGE gel to detect slower-migrating forms of Rad53. (Right) TCA-precipitated cell extracts from the same strains were analyzed in parallel.