Abstract
The regulated transport of proteins across the nuclear envelope occurs through nuclear pore complexes (NPCs), which are composed of >30 different protein subunits termed nucleoporins. While some nucleoporins are glycosylated, little about the role of glycosylation in NPC activity is understood. We have identified loss-of-function alleles of ALG12, encoding a mannosyltransferase, as suppressors of a temperature-sensitive mutation in the gene encoding the FXFG-nucleoporin NUP1. We observe that nup1Δ cells import nucleophilic proteins more efficiently when ALG12 is absent, suggesting that glycosylation may influence nuclear transport. Conditional nup1 and nup82 mutations are partially suppressed by the glycosylation inhibitor tunicamycin, while nic96 and nup116 alleles are hypersensitive to tunicamycin treatment, further implicating glycosylation in NPC function. Because Pom152p is a glycosylated, transmembrane nucleoporin, we examined genetic interactions between pom152 mutants and nup1Δ. A nup1 deletion is lethal in combination with pom152Δ, as well as with truncations of the N-terminal and transmembrane regions of Pom152p. However, truncations of the N-glycosylated, lumenal domain of Pom152p and pom152 mutants lacking N-linked glycosylation sites are viable in combination with nup1Δ, suppress nup1Δ temperature sensitivity, and partially suppress the nuclear protein import defects associated with the deletion of NUP1. These data provide compelling evidence for a role for glycosylation in influencing NPC function.
THE nuclear envelope (NE) of eukaryotic cells is composed of two concentric lipid bilayers, the inner nuclear membrane (INM) and the outer nuclear membrane (ONM). The ONM is contiguous with the endoplasmic reticulum (ER) and the space between the ONM and INM is equivalent to the ER lumen. The only site of contact between the ONM and INM is at the nuclear pore complexes (NPCs). NPCs form aqueous channels between the cytoplasm and nucleus, allowing passive diffusion across the NE of ions and other small molecules and regulated transport of larger molecules, including proteins and mRNAs. NPCs are composed of >30 different proteins termed nucleoporins (Nups), which are generally present in 8–32 copies per NPC (reviewed in Suntharalingam and Wente 2003).
Nup proteins provide both the structure and the transport function of NPCs. Most Nups occupy a discrete location along the nuclear/cytoplasmic axis within the NPC, with some Nups asymmetrically localized toward the cytoplasmic face, some Nups at the nucleoplasmic face, and some distributed symmetrically within the central core of the NPC (Rout et al. 2000). This asymmetry may allow specific Nups to function in specific stages of substrate translocation across the NPC (Pyhtila and Rexach 2003; Rout et al. 2003). Nups can be divided into two basic classes based on the presence or the absence of a conserved phenylalanine-glycine (FG) repeat sequence. The FG repeats function as low-affinity docking sites for soluble transport factors that facilitate the translocation across the NPC of cargoes containing nuclear localization signals (NLSs) or nuclear export signals. These transport factors, which include the karyopherin/importin/exportin family of Ran-binding proteins, associate directly with FG repeats and are dependent upon this association for translocation across the NPC (see Fahrenkrog et al. 2004).
FG Nups can be divided further into subclasses based on composition of their FG repeats. The cytoplasmically oriented Nup42p and Nup159p proteins contain conserved SAFG or PSFG sequences within each repeat (Gorsch et al. 1995; Stutz et al. 1995). These FG repeats associate with soluble transport factors, but are not essential for cell viability, despite their asymmetric distribution within the NPC (Kraemer et al. 1995; Del Priore et al. 1997; Hurwitz et al. 1998; Floer and Blobel 1999; Strawn et al. 2004). GLFG-Nups contain a glycine-leucine pair upstream of most FGs and seem to have the broadest substrate specificity. GLFG repeat sequences associate with most karyopherins (Kaps) and are localized symmetrically within the central region of the NPC (Rout et al. 2000; Allen et al. 2001). Removal of the GLFG region of individual GLFG-Nups in Saccharomyces cerevisiae does not affect cell viability or transport kinetics, but the disruption of specific combinations of GLFG regions from two or more GLFG-Nups results in lethality and/or decreased nuclear import rates (Strawn et al. 2004). Thus, some combination of GLFG repeats is necessary for efficient NPC function. FxFG repeats are found in Nup1p, Nup2p, Nup60p, and Nsp1p in yeast. These repeats associate with the essential heterodimeric karyopherin Kap95p/Kap60p, which functions to import classical NLS (cNLS)-containing proteins. Interestingly, cells lacking FxFG repeats from all four of these proteins are viable (Strawn et al. 2004) and a Nup1p domain without FGs also associates with Kap95p/Kap60p (Pyhtila and Rexach 2003), suggesting that the FxFG repeats are not essential for Kap95/Kap60-mediated protein import.
While FxFG repeats are not required for cell viability in yeast, the FxFG-repeat-containing Nup1 protein is essential in some genetic backgrounds (Davis and Fink 1990) and a loss of Nup1 is lethal at elevated temperatures in most others (Bogerd et al. 1994; Schlaich and Hurt 1995). Complete disruptions of NUP1 are also synthetically lethal with mutant alleles of NUP2 and NSP1 (Loeb et al. 1993), suggesting that the non-FG sequences of these Nups may carry out important overlapping functions. Nup1, Nup2, and Nup60 are localized to the nucleoplasmic face of the NPC where they associate transiently with the NPC basket, while Nsp1 is associated with the central region of the NPC (Rout et al. 2000). NUP1 deletion (nup1Δ) mutants are unable to efficiently export poly(A) RNA or to import cNLS-containing cargoes at elevated temperatures (Bogerd et al. 1994; Schlaich and Hurt 1995; Fischer et al. 2002). However, the role of Nup1p in these processes remains to be determined.
While many Nups contain FG repeats, over half of the nucleoporins do not exhibit FG repeat sequences. Both metazoan and yeast cells express non-FG-Nups that are integral membrane proteins, associating with the NPC via a cytoplasmic region, spanning the NE, and containing a domain within the NE lumen. The presence of a transmembrane domain allows these proteins to provide a physical link between the NPC and NE (see Suntharalingam and Wente 2003). In metazoans, gp210 is a membrane-spanning glycoprotein essential for NPC assembly (Wozniak et al. 1989; Cohen et al. 2003). The majority of gp210 is on the cisternal side of the NPC pore membrane, with only a short 60-amino-acid region exposed on the NPC side of the membrane (Greber et al. 1990). Both overexpression of this tail domain and RNAi-mediated depletion of gp210 inhibit NPC assembly (Drummond and Wilson 2002; Cohen et al. 2003). Expression of antibodies that associate with the lumenal domain of gp210 results in decreased nuclear import of NLS-containing proteins and a reduced diffusion rate across the pore of smaller proteins, implicating the glycosylated region of gp210 in NPC function (Greber and Gerace 1992). However, the role of glycosylation in NPC assembly and nucleocytoplasmic transport has not been examined.
In yeast, three transmembrane NPC proteins—Ndc1p, Pom34p, and Pom152p—have been identified and all three lack FG repeats. Ndc1p is a multipass transmembrane protein that localizes to both NPCs and the spindle pole body (Chial et al. 1998). Ndc1p function at the NPC is important for NPC assembly, but ndc1 mutants have not been observed to affect transport activity (Lau et al. 2004). Pom34p is a small, transmembrane NPC protein of unknown function (Rout et al. 2000). Pom152p, like gp210 in mammalian cells, is a large, transmembrane glycoprotein with the majority of its mass in the NE lumen (Wozniak et al. 1994; Tcheperegine et al. 1999). Despite encoding the only large integral membrane protein in the yeast NPC, POM152 is not essential (Wozniak et al. 1994). However, truncation mutants of POM152 lacking NPC and transmembrane domains exhibit synthetic lethal interactions with genes encoding several other NPC components, including Nup188p, Nup157p, Nup170p, Nup59p, and Nic96p (Aitchison et al. 1995; Tcheperegine et al. 1999). Of these Nups, only nup188 mutants also exhibit synthetic interactions with a truncation of the glycosylated lumenal domain of POM152 (Tcheperegine et al. 1999). The function of the Pom152p lumenal domain has not been determined. Here we provide genetic and functional evidence that the glycosylation of Pom152 influences NPC function.
MATERIALS AND METHODS
Reagents, strains, plasmids, and media:
Enzymes for molecular biology were purchased from New England Biolabs (Beverly, MA) and Sigma-Aldrich (St. Louis) and were used per manufacturer's instructions. Yeast transformations were performed as described (Woods and Gietz 2001) as were genetic manipulations, yeast cell culture, and media preparation (Guthrie and Fink 1991). Tunicamycin suppression was tested on YPD plates containing 0.5 or 1.0 μg/ml tunicamycin (Sigma-Aldrich). Plasmids pPom152-HA, p1-301, p170-1337, and pΔ176-263 were kindly contributed by R. Wozniak (University of Alberta). Plasmid KBB382 (CEN LEU2 pom152Δglyc) was generated by site-directed mutagenesis of pPom152-HA using oligonucleotides that altered codons encoding asparagines at residues 280, 398, 569, and 1099 to alanine. Mutagenesis was performed using the QuikChange multi-mutagenesis kit (Stratagene, La Jolla, CA) per manufacturer's instructions. Mutagenesis was confirmed by DNA sequencing using standard techniques. Yeast strains containing genomic deletions of NUP1, NUP188, NUP100, POM152, and ALG12 were purchased from OpenBiosystems (Huntsville, AL) and mated to produce strains used in this study (Table 1).
TABLE 1.
Yeast strains used in this study
| Strain name | Genotype | Source |
|---|---|---|
| BY4742 | MATα his3 leu2 lys2 ura3 | Open Biosystems |
| W303 | MATaade2 trp1 leu2 his3 ura3 | R. Rothstein |
| Y-728 | MATanic96-1∷ProtA∷LEU2 nic96ΔHIS3 ura3 ade2 trp1 | Zabel et al. (1996) |
| SWY29 | MATanup116-5ΔHIS3 ade2 ura3 his3 trp1 leu2 | Wente et al. (1992) |
| NUP82-Δ108 | MATα nup82ΔHIS3 leu2 lys2 trp1 ura3 + (pNup82Δ108 LEU2) | Hurwitz and Blobel (1995) |
| LGY101 | MATα rat7-1/nup159-1 his3 ura3 leu2 | Gorsch et al. (1995) |
| LDY461 | MATanup1ΔLEU2 ade2 trp1 leu2 his3 ura3 | This study |
| LDY563 | MATanup1ΔLEU2 nup100ΔURA3 trp1 his3 ade2 | This study |
| LDY572 | MATanup100ΔURA3 leu2 ade2 his3 trp1 | This study |
| LDY796 | MATα nup1ΔHIS3 ade2 trp1 leu2 ura3 | This study |
| KBY353 | MATα bnp1∷Tn3∷LEU2 nup1ΔHIS3 ade2 trp1 leu2 ura3 | This study |
| KBY423 | MATanup1ΔHIS3 ade2 trp1 leu2 ura3 | This study |
| KBY644 | MATα nup133ΔKAN his3 leu2 lys2 ura3 | Open Biosystems |
| KBY669 | MATanup1ΔHIS3 ade2 ura3 trp1 | This study |
| KBY671 | MATα nup1ΔHIS3 ecm39ΔKANR ade2 ura3 trp1 | This study |
| KBY673 | MATaecm39ΔKAN ura3 trp1 | This study |
| KBY675 | MATα ura3 his3 trp1 | This study |
| KBY776 | MATα pom152ΔKAN his3 leu2 ura3 lys2 | Open Biosystems |
| KBY795 | MATα nup170ΔKAN his3 leu2 lys2 ura3 | Open Biosystems |
| KBY1046 | MATα pom152ΔKAN his3 leu2 ura3 lys2+ (LDB59 CEN URA3 NUP1) | This study |
| KBY1047 | MATα nup1ΔKAN pom152ΔKAN his3 leu2 ura3 + (LDB59 CEN URA3 NUP1) | This study |
| KBY1048 | MATα nup1ΔKAN his3 leu2 ura3 + (LDB59 CEN URA3 NUP1) | This study |
| KBY1293 | MATα nup188ΔKAN his3 leu2 ura3 lys2 | Open Biosystems |
| KBY1329 | MATα pom152ΔKAN his3 leu2 ura3 lys2+ (KBB382 LEU2 pom152Δglyc) | This study |
| KBY1366 | MATα pom152ΔKAN his3 leu2 ura3 lys2+ (pRS315 CEN LEU2) | This study |
| KBY1374 | MATα nup1ΔKAN pom152ΔKAN his3 leu2 ura3 lys2+ (KBB382) | This study |
bnp suppressor screen:
Yeast strain LDY796 (Table 1) was transformed with a NotI-digested mTn∷LEU2 mutagenized yeast library, as described in Ross-Macdonald et al. (1997), and transformants were plated on SC −Leu media. Transformants were replica plated to SC −Leu media containing 1 mg/ml 5-fluoro-orotic acid (5-FOA) and incubated at 37°. Viable colonies were restreaked, mated to a W303 wild-type haploid, sporulated, and dissected. Resulting haploid spores were scored for cosegregation of Leu+ ts+ phenotypes. The genomic DNA immediately adjacent to the Tn insertion in bnp1 was identified using isolation of an integrated copy of the pRSQ2-URA plasmid as described previously (Ross-Macdonald et al. 1997) followed by sequencing of the isolated plasmid DNA. The presence of the transposon within genomic ALG12 was confirmed by PCR using primer pairs internal to the transposon and to ALG12 (data not shown).
Protein import and RNA export:
To examine protein import, strains KBY675 (wild type), KBY669 (nup1Δ), KBY671 (alg12Δ nup1Δ), KBY673 (alg12Δ), BY4742 (wild type), KBY1366 (pom152Δ), KBY1329 (pom152Δglyc), and KBY1374 (nup1Δ pom152Δglyc) were transformed with plasmid pSV40-NLS-GFP (Shulga et al. 1996), grown in SD −Ura to A600 0.05–0.2, and observed by direct fluorescence microscopy using a Nikon E600 epifluorescence microscope. Images were captured using a Hamamatsu (Tokyo) C5810 cooled CCD camera using National Institutes of Health Image software and were processed using Adobe Photoshop. In situ hybridization assays were performed on strains LDY675, KBY669, KBY671, and KBY673 as described previously (Amberg et al. 1992).
Analysis of pom152 mutants in nup1Δ:
Plasmids expressing truncated alleles of POM152 (pPom152-HA, p1-1219, p1-301 p170-1337, p1025-HA, and pΔ295-1036-HA; Tcheperegine et al. 1999) and KBB382 were transformed into strain KBY1047 [nup1Δ∷KANR pom152Δ∷KANR + pLDB59 (CEN URA3 NUP1)] and transformants were selected for on SC −Leu media. Transformants were streaked to SC −Leu media containing 1 μg/ml 5-FOA to select against pLDB59 (Boeke et al. 1987) and to SC −Ura media. Cells were incubated at 24°, 30°, and 37° for 2–5 days and scored for growth.
Protein extraction and Western blotting:
Yeast strains BY4742 or KBY673 containing pPom152-HA or KBB382 were grown to OD600 = 0.2–0.4 in 10 ml SD −Leu media. Cells were then lysed using acid-washed glass beads and precipitated using trichloroacetic acid as described (Adams et al. 1997). Cells treated with tunicamycin were incubated in SD −Leu containing 1.0 μg/ml tunicamycin for 3 hr before lysis. Protein extracts were electrophoretically separated on 7.5% polyacrylamide, transferred to nitrocellulose, and probed with 12CA5 anti-HA antibody (Babco, Berkeley, CA). Detection of bound antibody was performed using chemiluminescence (Bio-Rad, Hercules, CA).
RESULTS
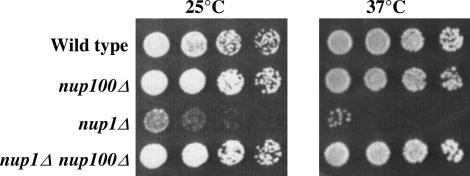
Cells lacking functional Nup1p have a temperature-sensitive growth phenotype and exhibit defects in mRNA nuclear export, nuclear protein import, and NE morphology (Davis and Fink 1990; Bogerd et al. 1994; Schlaich and Hurt 1995). While nup1Δ cells are dead at 37° and grow slowly at 25°, we observed that nup1Δ cells grown under either of these conditions spontaneously accumulate colonies in which the conditional or slow-growth phenotype is suppressed (Figure 1; see nup1Δ cells at 37°). These suppressors are not linked to NUP1 and arise at high frequency, suggesting that the reversions are due to loss-of-function mutations in loci distinct from NUP1. We have observed this “suppressor” phenotype in nup1-deleted cells from several different strain backgrounds (data not shown). One possible explanation for the frequent appearance of nup1Δ suppressors is that the loss of any one of a large number of other genes could allow the normal cellular requirement for NUP1 to be lost, effectively allowing the cell to bypass NUP1 function. To test whether decreased function of another NPC component could bypass the normal Nup1p requirement at elevated temperature, we generated double-mutant cells mutated for NUP1 and other Nups. Mutations in NSP1, NUP2, NUP82, NUP116, NUP157, and NUP170 are synthetically lethal with nup1Δ (Loeb et al. 1993; Kenna et al. 1996; Shulga et al. 1996; data not shown). However, nup1Δ nup100Δ cells grew better at 25° than nup1Δ cells and grew at a rate indistinguishable from wild type at 37° (Figure 1). We observed this suppression of nup1Δ by nup100Δ in both W303 and S288C yeast strain backgrounds (data not shown). Thus, a deletion of a nucleoporin can bypass the NUP1 requirement for cell viability at 37°.
Figure 1.
A nup100 deletion suppresses nup1Δ temperature sensitivity. Wild type (W303), nup100Δ (LDY572), nup1Δ (LDY461), and nup1Δ nup100Δ (LDY563) haploid cells were grown in liquid culture to midlog phase and fivefold serial dilutions were spotted on YPD. Plates were incubated at 25° for 3 days and at 37° for 2 days.
A deletion in ALG12 suppresses nup1Δ temperature sensitivity:
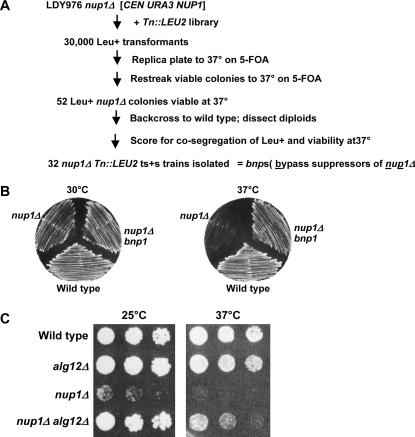
The high frequency at which suppressors spontaneously appeared in nup1Δ cells suggested that other loss-of-function mutations would also be able to bypass nup1Δ growth phenotypes. To identify other mutations that bypass nup1Δ, we performed a large-scale genetic screen for second-site mutations that suppress nup1Δ temperature sensitivity (Figure 2A). To this end, we performed random mutagenesis on a nup1Δ strain by insertion of a transposon 3 (Tn3)-disrupted yeast genomic library (Ross-Macdonald et al. 1997). We then assayed the colonies receiving Tn3-containing DNA insertions for viability at 37°, which would indicate suppression of the temperature-sensitive phenotype of the nup1Δ parent strain. Those Tn3-containing strains that grew at 37° were backcrossed with the nup1Δ parent strain and the resulting diploids were dissected to confirm linkage of a single transposon with the suppressor phenotype in each mutant (see materials and methods). In this way, we identified 32 bypass suppressor of nup1Δ (bnp) mutants, which we have labeled bnp1–bnp32.
Figure 2.
Mutations in ALG12 suppress nup1Δ temperature sensitivity. (A) Flow diagram describing the genetic screen used to identify bypass suppressors of nup1Δ (bnp's). (B) Bypass suppressors of nup1Δ temperature sensitivity (bnp's) were generated as described in materials and methods. Wild type (W303), nup1Δ (LDY796), and nup1Δ bnp1 (KBY353) cells were streaked to YPD at 30° and at 37° and incubated for 54 hr. bnp1 is a truncation allele of ALG12 generated by Tn mutagenesis. (C) Log-phase cultures of wild type (W303), alg12Δ (KBY673), nup1Δ (KBY669), and nup1Δ alg12Δ (KBY671) cells were serially diluted and plated on YPD. Images were taken after 96 hr at 25° and after 56 hr at 37°.
Here we report the characterization of the bnp1 transposon insertion mutant. In this isolate, the presence of the transposon suppressed the temperature sensitivity of the nup1 deletion, restoring growth at 37° to nearly the rate of wild-type cells (Figure 2B). To identify the gene disrupted by the transposon in the bnp1 strain, we isolated DNA adjacent to the site of transposon insertion using plasmid rescue (Ross-Macdonald et al. 1997) and sequenced the DNA of the rescued, Tn-containing plasmid. In our bnp1 mutant, the transposon had inserted within the coding region of the ALG12 gene, resulting in truncation of the Alg12 protein after only 30 amino acids. Thus bnp1 is a severe truncation allele of ALG12 (ALG, asparagine-linked glycosylation; Burda et al. 1999). ALG12 was first identified in a screen for cells resistant to calcofluor (Lussier et al. 1997) and encodes an ER-localized α-16-mannosyltransferase required for synthesis of Man8GlcNAc2 from Man7GlcNAc2 (Burda et al. 1999).
Although the mutagenic transposon is inserted within the ALG12 gene, a small region of the endogenous Alg12p may still be produced. To confirm that the suppression of nup1Δ that we observed in bnp1 was the result of an alg12 loss-of-function mutation, we mated nup1Δ and alg12Δ haploid cells containing complete disruptions of each gene. We then sporulated and dissected the heterozygous diploids to isolate nup1Δ alg12Δ double-mutant haploid cells and compared the growth of alg12Δ nup1Δ cells with alg12Δ and nup1Δ single mutants (Figure 2C). Both wild-type and alg12Δ cells grew at similar rates at all temperatures tested, while nup1Δ cells grew slowly at 25° and were inviable at 37°. Importantly, nup1Δ alg12Δ double mutants grew at a rate indistinguishable from wild type at 25° and significantly better than nup1Δ at 37°. These data indicate that a loss-of-function alg12 mutant can function as a bypass suppressor of nup1Δ temperature sensitivity.
alg12Δ suppresses the nuclear protein import defect exhibited in nup1Δ:
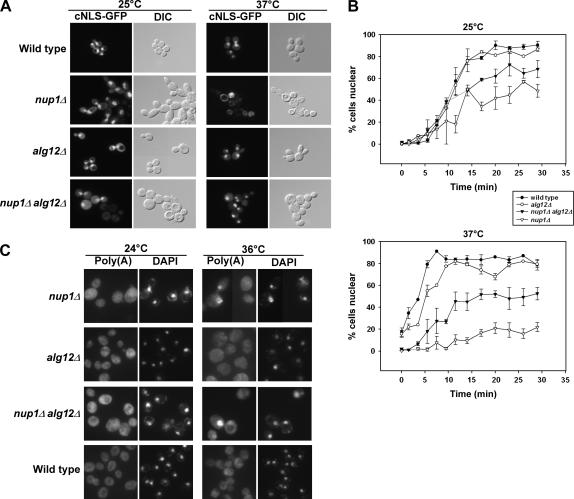
Nup1p is essential both for efficient nuclear import of cNLS-containing proteins and for export of polyadenylated RNAs (Davis and Fink 1990; Bogerd et al. 1994; Schlaich and Hurt 1995; Fischer et al. 2002). Because an alg12Δ mutation can suppress the growth phenotype associated with a deletion of nup1, we investigated whether alg12Δ mutants influence nuclear transport kinetics, both in cells that are otherwise wild type and in nup1Δ cells. To examine whether protein import is altered in alg12Δ and nup1Δ alg12Δ cells, we examined the intracellular localization of green fluorescent protein (GFP)-tagged nuclear import substrates in alg12Δ mutant cells. As a nuclear import substrate, we used cNLS-GFP, containing a “classical” NLS imported by the Kap95p/Kap60p heterodimer (Shulga et al. 1996). Nup1p associates directly with Kap95p/Kap60p and is essential for efficient cNLS import (Booth et al. 1999; Pyhtila and Rexach 2003). We expressed cNLS-GFP in wild-type, nup1Δ, alg12Δ, and nup1Δ alg12Δ cells and observed the steady-state localization of the cNLS-GFP reporter in live cells by fluorescence microscopy. Cells were either incubated at 25° or shifted from 25° to 37° for 3 hr before assaying. All four strains exhibited predominantly nuclear accumulation of cNLS-GFP at 25° (Figure 3A). However, both nup1Δ and nup1Δ alg12Δ cultures contained some cells (<25%) with predominantly cytoplasmic GFP. In cells shifted to 37°, nearly all of the wild-type and alg12Δ cells accumulated nuclear cNLS-GFP, while the nup1Δ alg12Δ double mutant again had >70% of cells with nuclear fluorescence. Only the nup1Δ cells showed a severe defect in nuclear cNLS-GFP import, with <10% of cells accumulating GFP within the nucleus. These observations suggest that a deletion of alg12 can suppress the severe cNLS import defect exhibited by nup1Δ cells, providing a possible mechanism by which alg12Δ suppresses nup1Δ temperature sensitivity.
Figure 3.
Deletion of ALG12 suppresses nup1Δ protein import defects but not poly(A)+ RNA export defects. (A) Wild-type (KBY675), nup1Δ (KBY669), alg12Δ (KBY673), and nup1Δ alg12Δ (KBY671) cells expressing a cNLS fused to GFP (Shulga et al. 1996) were grown to early log phase at 25° and then retained at 25° or shifted to 37° for 3 hr. Cells were observed by direct fluorescence (cNLS-GFP) or differential interference contrast microscopy. (B) Wild-type (KBY675), nup1Δ (KBY669), alg12Δ (KBY673), and nup1Δ alg12Δ (KBY671) cells were examined for cNLS-GFP import kinetics (Shulga et al. 1996; see materials and methods). Briefly, cells expressing cNLS-GFP were treated with 2-deoxyglucose and sodium azide for 1 hr, washed, and then assayed for cNLS reimport by fluorescence microscopy. Import rate was determined by plotting the percentage of cells exhibiting nuclear fluorescence of cNLS-GFP vs. time. (Top) Relative cNLS-GFP import rates in cells grown at 25°. (Bottom) Cultures shifted to 37° for 3 hr and retained in 37° media throughout the import assay. Data represent mean values from at least three independent experiments. Error bars represent standard error of the mean. (C) Wild-type, nup1Δ, alg12Δ, and nup1Δ alg12Δ cells were grown to early log phase at 24° and then incubated at 24° or shifted to 36° for 3 hr. Cells were fixed, permeabilized, and incubated with a digoxigenin-conjugated oligo(dT)50 probe. Hybridized probe was detected using FITC-associated antidigoxigenin antibodies (Amberg et al. 1992). “Poly(A)” represents localization of polyadenylated RNA, as detected by the oligo(dT) probe. “DAPI” indicates the location of DAPI-stained nuclei in the same cells.
While these data indicate that alg12Δ nup1Δ cells can import cNLS-GFP, they do not address the relative rate of cNLS nuclear import in each strain. To determine if an alg12 deletion alters the rate of cNLS-GFP import, we performed a kinetic nuclear import assay (Shulga et al. 1996). At 25°, both wild type and alg12Δ cells imported the cNLS-GFP reporter at equivalent rates, with ∼50% of cells exhibiting nuclear cNLS-GFP accumulation within 11 min after removal of metabolic inhibitors that effectively abolish active nucleocytoplasmic transport (Figure 3B). In contrast, only ∼20% of nup1Δ cells accumulated the cNLS reporter in the nucleus after an equivalent amount of time. This slowed rate of cNLS-mediated import was partially suppressed by a deletion of ALG12, as 42% of alg12Δ nup1Δ cells accumulated nuclear cNLS-GFP after 11 min. At 37°, alg12Δ cells exhibited slightly slower cNLS import kinetics than did wild-type cells, with ∼5 min required to accumulate nuclear fluorescence in 50% of alg12Δ cells compared to 3.5 min in wild type. In contrast, nup1Δ cells nearly entirely lacked cNLS import with <20% of cells accumulating nuclear cNLS-GFP, even 30 min after removal of metabolic inhibitors. alg12Δ nup1Δ cells had an intermediate rate of cNLS-GFP import, with 50% of cells containing nuclear GFP after ∼17 min. Thus, deletion of ALG12 partially suppresses the cNLS-GFP import defect associated with the loss of NUP1.
Cells lacking Nup1p also exhibit a reduction in nuclear poly(A)+ RNA export (Bogerd et al. 1994; Schlaich and Hurt 1995; Fischer et al. 2002). To test for the influence of alg12 deletions on mRNA export, we examined poly(A)+ RNA localization in cells lacking Alg12p using in situ hybridization with an oligo(dT) probe (Amberg et al. 1992). None of the nup1Δ, alg12Δ, or nup1Δ alg12Δ mutant cells exhibited an accumulation of poly(A)+ RNA in the nucleus when grown at the permissive temperature (Figure 3C). However, after a 3-hr shift to 36°, some nup1Δ cells exhibited clear nuclear poly(A)+ RNA localization, in agreement with the partial RNA export defect observed previously for nup1 mutants (Bogerd et al. 1994; Schlaich and Hurt 1995; Fischer et al. 2002). nup1Δ alg12Δ double-mutant cells also exhibited a nuclear accumulation of poly(A)+ RNA in the nucleus, similar to that seen for nup1Δ. In contrast, wild-type and alg12Δ cells did not accumulate poly(A)+ RNA in the nucleus. These data suggest that the deletion of alg12Δ does not suppress the mRNA export defect of nup1Δ cells.
Decreased glycosylation suppresses nup1Δ temperature sensitivity:
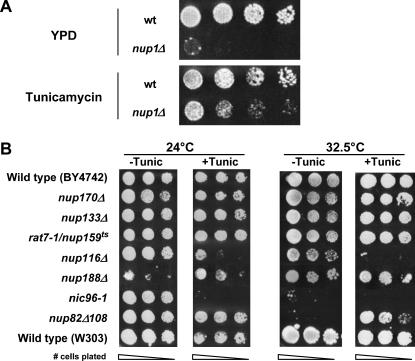
Alg12p is a mannosyltransferase required for normal assembly of lipid-associated polysaccharide chains in the ER. Once assembled, these branched chains are then transferred to specific asparagine (N) residues on proteins within the ER lumen. This “N-linked” glycosylation occurs on most proteins that enter the ER and the presence of these branched carbohydrate chains is essential for the stability, localization, and function of many glycosylated proteins (reviewed in Helenius and Aebi 2001). Since Alg12p is important for normal glycosylation, we tested to see if inhibiting glycosylation in vivo would alter the phenotypes associated with mutations in NUP1. To this end, we plated serially diluted cultures containing nup1Δ and wild-type cells on tunicamycin, an inhibitor of protein glycosylation (Mahoney and Duksin 1979), and assayed for growth at temperatures ranging from 24° to 37°. We observed that both wild-type and nup1Δ cells failed to grow at any temperature in the presence of 2.0 μg/ml tunicamycin (data not shown). However, cells exposed to lower doses of tunicamycin remained viable but had slowed growth kinetics. We observed that wild-type cells grown at 30° for 48 hr in the presence of 0.5 μg/ml tunicamycin exhibited a slightly decreased growth rate compared to cells incubated on YPD (Figure 4A). In contrast, nup1Δ cells incubated under the same conditions grew significantly better in the presence of tunicamycin than on YPD. A similar increase in growth in the presence of 0.5 μg/ml tunicamycin was observed for nup1Δ cells incubated at 28° and 32.5° (data not shown). Thus, a general decrease in glycosylation in vivo partially suppresses the temperature-sensitive phenotype of nup1Δ cells.
Figure 4.
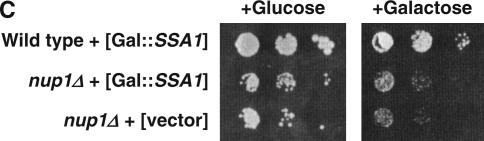
Inhibition of glycosylation alters the growth rate of nup1, nup116, nup82, and nic96 cells. (A) Wild-type (W303) and nup1Δ (KBY423) cells were grown to log phase in YPD at 25° and then serially diluted and plated on YPD and YPD containing 0.5 μg/ml tunicamycin. Plates were incubated at 30° for 72 hr. (B) Overnight cultures of wild-type (W303 and BY4742), nup170Δ (KBY795), nup133Δ (KBY644), rat7-1/nup159ts (LGY101), nup188Δ (KBY1293), nup116Δ (SWY29), nic96-1 (Y-728), and nup82Δ108 (NUP82-Δ108) cells were grown to log phase, resuspended at identical concentrations, serially diluted, and plated on YPD (−Tunic) or on YPD containing 1.0 μg/ml tunicamycin (+Tunic). Plates were incubated at 24° and 32.5° for 72 hr. (C) Wild-type (BY4742) and nup1Δ (LDY461) cells were transformed with pGAL∷SSA1 (2μ LEU2 GAL∷SSA1) or pRS315 (CEN LEU2). Cells were grown to log phase and serial dilutions were spotted onto YPD media containing glucose or galactose. Plates were incubated at 32.5° for 72 hr.
To determine if the glycosylation-dependent growth defects that we observed were specific to nup1Δ, we incubated additional NPC mutants on media containing tunicamycin. A tunicamycin concentration of 1.0 μg/ml strongly suppressed the conditional growth of nup82Δ108 at 32.5° (Figure 4B) and at 37° (data not shown), but did not significantly alter the growth of nup133Δ, nup188Δ, nup159ts, nup100Δ, nup2Δ, nup53Δ, or nup59Δ cells compared to wild type (Figure 4B; data not shown). Interestingly, nup116Δ, nic96-1, and nic96-2 (Figure 4B; data not shown) mutants exhibited hypersensitivity to tunicamycin. These data suggest that glycosylation influences a distinct subset of Nups and that different Nup proteins may have distinct functional interactions with glycosylated proteins.
The inhibition of glycosylation by tunicamycin has been shown previously to induce the unfolded protein response (UPR) for elimination of misfolded ER proteins (Travers et al. 2000). One consequence of UPR induction is an increase in expression of some protein chaperones and their cofactors. Overexpression of Ssa1p, a cytosolic yeast hsp70, has been shown previously to suppress defects in cNLS-mediated nuclear import (Shulga et al. 1996, 1999). To ascertain whether the suppression of nup1Δ temperature sensitivity that we observed was the result of increased Ssa1p, we overexpressed SSA1 in wild-type and nup1Δ cells and assayed for growth at permissive and nonpermissive temperatures (Figure 4C). The nup1Δ cells did not exhibit a detectable change in growth at either temperature upon overexpression of Ssa1p, suggesting that suppression of nup1Δ by tunicamycin is not simply due to an UPR-induced increase in Ssa1p activity.
nup1Δ cells exhibit allele-specific interactions with pom152 mutants lacking N-linked glycosylation sites:
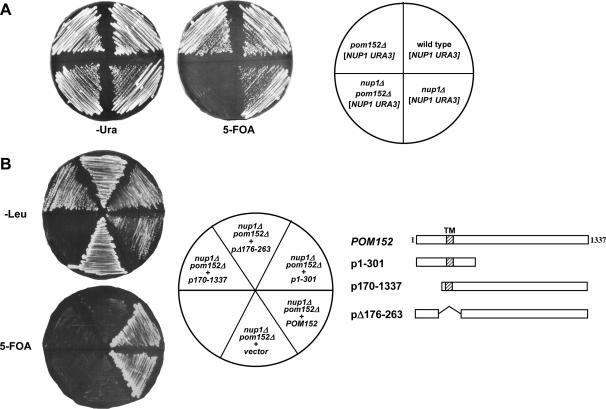
Since N-linked glycosylation occurs in the ER lumen, most NPC proteins are unlikely to be glycosylated. However, transmembrane proteins that link the NPC to the NE span the lipid bilayer and extend into the lumenal space between the inner and outer nuclear membranes. This space is contiguous with the ER lumen, allowing transmembrane Nups to be glycosylated on their lumenal domains. Indeed, Pom152p is a type II transmembrane nucleoporin whose C-terminal domain is within the NE lumen and is glycosylated at from one to four asparagine residues (Tcheperegine et al. 1999). Given the link between glycosylation and Nup1p function that we had observed, we examined whether nup1 and pom152 mutants exhibited genetic interactions. To this end, we generated wild-type, pom152Δ, nup1Δ, and pom152Δ nup1Δ haploid mutant cells containing a NUP1 URA3 plasmid (Figure 5A). We then tested each strain for growth on SD −Ura media, in which the plasmid-borne NUP1 is retained, and on 5-FOA, which selects against the URA3-containing NUP1 plasmid. A deletion of POM152 is not lethal and does not confer a detectable growth phenotype (Wozniak et al. 1994). As expected, cells lacking NUP1 exhibited a temperature-sensitive phenotype, growing slowly at 25°, but failing to grow at 37°. Surprisingly, cells lacking both NUP1 and POM152 were inviable at all temperatures. Thus, nup1Δ pom152Δ mutants are synthetically lethal, suggesting a functional interaction between the two gene products.
Figure 5.
nup1Δ growth defects are suppressed by pom152 alleles lacking glycosylation. (A) Wild-type (S288C), pom152Δ (KBY1046), nup1Δ (KBY1048), and pom152Δ nup1Δ (KBY1047) cells containing pLDB59 (CEN URA3 NUP1) were streaked on SD −Ura and 5-FOA media, incubated at 25° for 4 days, and scored for growth. (B) nup1Δ pom152Δ (KBY1047) cells containing a CEN URA3 NUP1 plasmid were transformed with plasmids expressing full-length POM152, deletions of N-terminal (p170-1337), transmembrane (pΔ176-263), and lumenal (p1-301) Pom152 domains (Tcheperegine et al. 1999), and empty vector (pRS315). Transformants were streaked on SD −Leu and 5-FOA and incubated at 24°. Streaks were scored for growth after 3 days on SD −Leu and after 5 days on 5-FOA. (C) POM152, pom152ΔGlyc (KBB382), pom152ΔC (p1-301), and empty vector (pRS315) were transformed into nup1Δ pom152Δ (KBY1047) cells containing a CEN URA3 NUP1 plasmid. Transformants were streaked to SD −Leu and 5-FOA and incubated at 24° and at 37°.
The synthetic lethality between nup1Δ and pom152Δ was somewhat unexpected, given our observation that a decrease in glycosylation actually increases growth rates of nup1Δ mutants. We predicted that the synthetic lethality between NUP1 and POM152 was the result of the dependence of nup1Δ mutants on a region of Pom152p other than the glycosylated residues. Other Nups that are synthetically lethal with pom152 exhibit allele-specific synthetic interactions with pom152 mutants lacking specific sequences (Tcheperegine et al. 1999). To determine if nup1Δ is synthetically lethal with specific deletions of Pom152p domains, we tested whether several pom152 truncation alleles (Tcheperegine et al. 1999) were viable in combination with a deletion of nup1Δ. To this end, nup1Δ pom152Δ cells containing a URA3 NUP1 plasmid were transformed with plasmids expressing N-terminal (p170–1337), transmembrane domain (pΔ176-263), and C-terminal (p1-301) deletions of POM152. Transformants were then plated on 5-FOA-containing media to select against the URA3 NUP1 plasmid and were scored for growth at 24° and 37° (Figure 5B). At 24° nup1Δ cells containing N-terminal or transmembrane deletions of POM152 failed to grow, indicating that cells lacking Nup1p require both the NPC domain and the transmembrane domain of Pom152p for viability. However, nup1Δ cells expressing a pom152 mutant lacking the C-terminal 1036 residues (p1-301, hereafter referred to as pom152ΔC) grew at a rate indistinguishable from nup1Δ cells expressing full-length Pom152p. These data indicate that only the N-terminal 301 amino acids of Pom152p, including the NPC and transmembrane domains, are essential for complementation of nup1Δ pom152Δ synthetic lethality while the lumenal C-terminal domain is not. At 37°, the N-terminal and transmembrane truncations were again inviable in a nup1Δ background (data not shown). As expected, nup1Δ cells expressing full-length POM152 were also inviable, since the nup1Δ is temperature sensitive. However, cells expressing a C-terminal truncation of Pom152p grew at 37° in the absence of Nup1p (Figure 6B), indicating that the loss of the lumenal, glycosylated Pom152p domain can suppress the temperature sensitivity of a nup1 deletion.
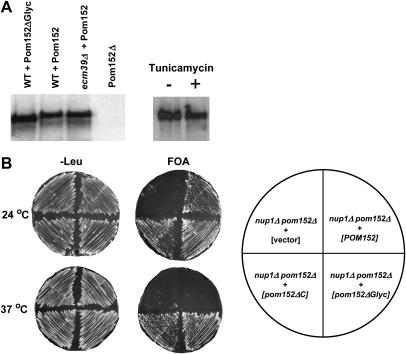
Figure 6.
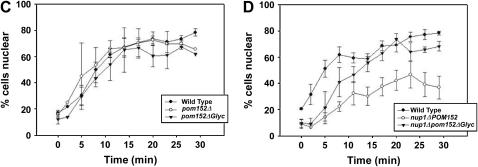
Elimination of Pom152p glycosylation suppresses nup1Δ phenotypes. (A) Protein extracts from wild type and alg12Δ cells expressing HA-tagged Pom152Δglyc or Pom152p were run on 7.5% polyacrylamide and probed with anti-HA antibody. (Left) The electrophoretic mobility of Pom152Δglyc compared to Pom152p in each strain. (Right) The relative mobility of Pom152p extracted from cells treated with 1.0 μg/ml tunicamycin (+) vs. Pom152p from untreated (−) cells. (B) KBY1047 (nup1Δ pom152Δ + CEN URA3 NUP1) cells were transformed with plasmids containing empty vector (pRS315), POM152 (pPom152-HA), pom152ΔC (p1-301), or pom152Δglyc (KBB382). Transformants were streaked on SD −Leu and 5-FOA and incubated at 24° and at 37°. (C) Wild-type (BY4742), pom152Δ (KBY1336), and pom152Δglyc (KBY1329) cells expressing cNLS-GFP were assayed for import of a cNLS-GFP reporter after release from metabolic arrest at 37° (Shulga et al. 1996). The percentage of cells exhibiting nuclear fluorescence is plotted against the time elapsed after release from arrest. Data represent mean values from at least three independent experiments. Error bars represent standard error of the mean. (D) Wild-type (BY4742), nup1Δ (KBY1376), and nup1Δ pom152Δglyc (KBY1374) cells were examined for cNLS-GFP import kinetics as described above. Kinetic assays were performed after incubation at 37° for 3 hr.
The suppression of nup1Δ by pom152ΔC may be the consequence of removing Pom152p glycosylation sites or the result of truncating an important Pom152p polypeptide sequence. Pom152p is glycosylated on Asp280 and at up to three other lumenal asparagines (Wozniak et al. 1994; Tcheperegine et al. 1999). On the basis of the consensus glycosylation sequence NXS/T, potential glycosylation sites also exist at N398, N569, and N1099 within the lumenal domain of Pom152p. To determine if a pom152 allele lacking glycosylation confers a nup1Δ suppressor phenotype, we performed site-directed mutagenesis on all four predicted N-linked glycosylation sites present in the C-terminal domain of Pom152p, converting each asparagine to an alanine. We confirmed the generation of pom152N280A, N398A, N569A, N1099A (pom152Δglyc) by DNA sequencing (data not shown) and by observing a change in electrophoretic mobility of the Pom152Δglyc protein relative to wild-type Pom152p (Figure 6A). Interestingly, Pom152p extracted from alg12Δ cells or cells treated with 1 μg/ml tunicamycin did not exhibit a detectable increase in electrophoretic mobility, suggesting that Pom152p retains at least a significant proportion of its glycosylated residues under these conditions.
Since pom152Δglyc is not glycosylated, we used this mutant to test whether the loss of Pom152p glycosylation suppresses nup1Δ. To this end, pom152Δglyc was expressed in nup1Δ pom152Δ cells and the growth phenotype of nup1Δ pom152Δglyc was compared with nup1Δ, nup1Δ pom152Δ, and wild-type cells at 24° and 37° (Figure 6B). nup1Δ pom152Δglyc cells grew faster than nup1Δ at 24°, at a rate similar to nup1Δ pom152ΔC. At 37°, both nup1Δ pom152ΔC and nup1Δ pom152Δglyc remained viable, while nup1Δ cells were unable to grow. Thus, elimination of Pom152p glycosylation suppresses the temperature-sensitive growth of nup1Δ.
To determine if the suppression of nup1Δ temperature sensitivity by Pom152Δglyc is due to enhanced nuclear protein import, we performed kinetics assays of protein import on pom152 and nup1 mutant cells. We first examined the rate of cNLS-GFP nuclear import in wild-type cells compared with cells lacking Pom152p and cells containing pom152Δglyc as their only source of Pom152p. Neither the total loss of Pom152p nor the removal of the lumenal glycosylation sites from Pom152p significantly alters the nuclear import kinetics of the cNLS-GFP reporter at 24° (data not shown) or at 37° (Figure 6C). We then examined the influence of Pom152p glycosylation on nuclear import in nup1Δ cells. The removal of the C-terminal Pom152p glycosylation sites does not significantly alter the rate of cNLS-GFP nuclear import in nup1Δ cells grown at 24° (data not shown). However, the expression of Pom152Δglyc in a nup1Δ strain incubated at 37° increases the kinetics of cNLS-GFP import to a rate intermediate to that observed for wild-type and nup1Δ cells (Figure 6D). These data indicate that the loss of Pom152p lumenal glycosylation alone does not significantly alter cNLS-mediated protein import under the conditions examined, but that eliminating N-linked glycosylation of the lumenal domain of Pom152p does partially suppress the conditional nuclear import defect observed in nup1Δ mutants, thus providing a likely mechanism for the suppression of nup1Δ temperature sensitivity by pom152Δglyc.
DISCUSSION
Here we provide strong evidence that glycosylation within the lumen of the NE influences NPC function. We show that the temperature-sensitive growth phenotype associated with a deletion of NUP1 is suppressed both by deletion of ALG12, a mannosyltransferase essential for normal glycosylation, and by treatment of cells with the glycosylation inhibitor tunicamycin. In addition, alg12Δ partially restores cNLS import in nup1Δ mutants. Finally, while nup1Δ is synthetically lethal with truncation of the N terminus or deletion of the transmembrane domain of Pom152p, eliminating glycosylation of the lumenal C terminus of Pom152p suppresses the temperature sensitivity and nuclear transport defects of nup1Δ. Together, these data strongly suggest that glycosylation of the lumenal domain of Pom152p plays a role in facilitating NPC function.
Despite the presence of glycosylated integral membrane proteins in the NPC, the relationship between glycosylation and NPC function remains unclear. In mammalian systems, treatment of cells with the lectin wheat germ agglutinin inhibits nuclear protein import (Finlay et al. 1987). NPCs that lack O-linked N-acetylglucosamine (GlcNAc) are unable to import karyophilic proteins or to dock Kap/cargo complexes at the cytoplasmic face of the NPC, even though the NE and major NPC structural components assemble appropriately in their absence (Finlay and Forbes 1990). These O-linked GlcNAc residues are present on the cytoplasmic surface of FG-Nups and may play a role in substrate docking and translocation through the NPC (Davis and Blobel 1987; Finlay and Forbes 1990; Greber and Gerace 1992; Miller et al. 1999) or may regulate the phosphorylation state of specific O-glycosylated Nups (Miller et al. 1999). Interestingly, O-linked GlcNAc modification of yeast Nups has not been reported.
In contrast to O-linked modifications, N-linked glycosylation may play a global role in NPC function, as both metazoan gp210 and yeast Pom152p are glycosylated at asparagine residues within the lumen of the NE (Wozniak et al. 1989; Tcheperegine et al. 1999). Exposure of cells to an antibody that associates with a lumenal region of gp210 inhibits nuclear protein import and decreases the apparent rate of diffusion through the NPC, suggesting that the lumenal domain of gp210 is essential for normal NPC function (Greber and Gerace 1992). However, the role of glycosylation of the lumenal gp210 domain in NPC function has not been examined. The importance of glycosylation in Pom152p function is equally unclear. The C-terminal glycosylated region of Pom152p is essential for viability in the absence of Nup188p, but this domain is not necessary for complementation of pom152Δ synthetic lethality with nic96, nup170, nup59 (Tcheperegine et al. 1999), or nup1 mutants (Figure 5B), suggesting that a subset of nucleoporins may interact functionally with the lumenal Pom152p region. Our observation that tunicamycin treatment alters the growth rate of some nup mutants [including nup1 and nic96 alleles, which interact genetically with pom152 (Tcheperegine et al. 1999; this study)], while inhibiting glycosylation does not detectably influence the growth of other nup deletions (including nup170Δ and nup59Δ), provides further evidence that a subset of Nups is influenced by lumenal glycosylation. Together, these data provide the first strong evidence that the glycosylation state of a nucleoporin can influence NPC function.
How might glycosylation influence or regulate NPC activity to produce the phenotypes observed in our mutants? Potential alterations in NPC function manifested in these phenotypes could include changes in active transport across the NPC, changes in NPC structure, alterations in NPC assembly, or some combination of the above. Our observation that cNLS-GFP import is enhanced in nup1Δ alg12Δ and nup1Δ pom152Δglyc cells compared to nup1Δ cells suggests that changes in glycosylation may directly influence active transport mechanisms. These changes may be specific to nucleocytoplasmic protein transport, as the mRNA export defect observed in nup1Δ is not suppressed by alg12Δ. In metazoan cells, antibodies directed against the domain of gp210 within the NE lumen also alter transport kinetics of a nucleophilic reporter protein, resulting in a decreased nuclear import rate (Greber and Gerace 1992). Some sec mutants also exhibit decreased nuclear import of cNLS-containing proteins (Nanduri et al. 1999), suggesting that appropriate ER function may be important for normal nucleocytoplasmic transport. Interestingly, the exposure of cells to tunicamycin does not seem to detectably alter the import kinetics of cNLS-containing reporter proteins (A. Gupta and K. Belanger, unpublished results).
Protein stability, intracellular targeting, and protein-protein interactions are influenced by the glycosylation state, especially within the endomembrane system (reviewed in Helenius and Aebi 2001). Changes in glycosylation of specific nucleoporins, such as Pom152p, could thus alter the relative number of various Nups at the NPC through any of these mechanisms, leading to changes in the number of Pom152p-associated Nups at the pore. These changes would likely alter the stoichiometry of Nups at each NPC, resulting in altered function. Our observation that a deletion of POM152 is synthetically lethal with nup1Δ while pom152 alleles lacking glycosylation sites suppress nup1Δ temperature sensitivity suggests that a reduction in Pom152p glycosylation does not lead to a loss of functional Pom152p protein at the NPC. In addition, we observe that wild-type Pom152p and Pom152ΔGlyc protein are present at similar levels (Figure 6A), suggesting that Pom152ΔGlyc is not being rapidly degraded. Instead, changes in the glycosylation state of Pom152p may alter NPC assembly and/or structure. The transmembrane nucleoporins Pom152p and Ndc1p may assemble early in NPC biogenesis to generate a precursor structure on which NPCs are formed (Marelli et al. 2001). Indeed, an Ndc1p allele that is defective in NPC assembly has recently been identified (Lau et al. 2004). Conditional alleles encoding the Pom152p-associated nucleoporin Nic96p result in a decrease in NPC number under growth at the nonpermissive temperature (Gomez-Ospina et al. 2000). While changes in NPC structure and number have not been assessed in pom152 mutant cells, the deletion of pom152 does not alter the rate of diffusion through the NPC of several GFP-tagged reporter proteins (Shulga et al. 2000), indicating that NPC structure and/or number is not altered significantly enough in the absence of Pom152p to affect passive transport through the nuclear pore.
The genetic screen reported here has identified up to 32 complementation groups that suppress the temperature sensitivity of a nup1Δ mutant. Since these mutants were generated by integrating a transposon insertion library into the nup1Δ starting strain, most of these mutations are presumably loss-of-function mutants created by the disruption of an open reading frame with the transposon. How might the generation of a second-site “deletion” result in the suppression of nup1Δ temperature sensitivity? One possibility is that the loss of Nup1p at the nuclear basket of the NPC slows the translocation of importin/cargo complexes through the NPC. The accumulation of these complexes within the nuclear basket may create a steric block within the NPC channel, resulting in the loss of translocation of some essential nucleophilic substrates across the NE. Suppression could then occur through the loss of some upstream step within the same pathway, preventing the importin/cargo complexes from ever entering the NPC and thus preventing an accumulation of importin/cargo within the NPC. Alternatively, suppression could be the result of a change in a second pathway that enhances translocation through the NPC, potentially via a Nup1p-independent pathway. In either case, some of the suppressor mutations are likely not to be alleles of genes that encode direct constituents of a pathway that mediates translocation (i.e., Nups or Kaps), but instead encode proteins that regulate the activity of factors directly involved in transport across the NPC.
Our results provide evidence that the glycosylation of NPC components influences NPC function, possibly by inhibiting a transport step upstream of Nup1p, enhancing a parallel import pathway that does not require Nup1p, or altering the structure of the NPC in such a way that cNLS import is enhanced in a nup1Δ mutant. The large number of spontaneous suppressors that we observed and the Tn-induced bnps that we have isolated support the likelihood that proteins other than nucleoporins and the relatively small number of soluble nuclear transport factors can influence NPC function and/or structure. A similarly large number of mutants were isolated in a genetic screen for mutants in NPC assembly and structure (Ryan and Wente 2002). In this screen, EMS mutagenesis of cells expressing Nup-GFP reporters generated up to 87 npa (NPC assembly) complementation groups in which several Nups, including Pom152p, are mislocalized at elevated temperatures. While some of these npa mutants encode alleles of the Ran-GTPase and its regulators (Ryan et al. 2003), several mutants also encode factors important for ER function (Ryan and Wente 2002), and most of the npa mutations remain unidentified. Each of the sec mutants identified in this screen produced aberrant ER structures, suggesting that abnormal proliferation of the ER (and thus of the NE) may result in aberrant NPC assembly. However, it remains possible that these mutants, either directly or indirectly, influence the efficiency of glycosylation in the ER and that the failure of Pom152p and other Nups to appropriately incorporate into the NPC is a consequence of changes in the glycosylation of Pom152p or other ER/NE components. The cloning of additional bnp's, npa's, and other mutations influencing NPC function will likely identify new signaling pathways and/or post-translational modifications that regulate NPC activity.
Here we have provided evidence that glycosylation is important for NPC function, at least in part through the post-translational modification of the lumenal domain of Pom152p. Future work will examine whether changes in the glycosylation state of Pom152p influence transport kinetics through the NPC and/or alter the composition or assembly of NPCs. Since the two additional transmembrane nucleoporins in yeast, Pom34p and Ndc1p, also contain predicted glycosylation sites in their lumenal domains, further investigation of their role in nuclear transport and assembly may also reveal insights into the regulation of NPC activity by glycosylation.
Acknowledgments
The authors are grateful to Rick Wozniak, Susan Wente, Ed Hurt, Michael Snyder, and David Goldfarb for generously sharing yeast strains and plasmids. We also thank Susan Wente for helpful comments on this manuscript and Karyn Goudie Belanger and Ajit Nott for technical assistance. This work was supported by National Institutes of Health grant GM-65107 to K.D.B. and Colgate University summer undergraduate research fellowships to K.M.M. and A.G.
References
- Adams, A., D. E. Gottschling, C. A. Kaiser and T. Stearns, 1997. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Aitchison, J. D., M. P. Rout, M. Marelli, G. Blobel and R. W. Wozniak, 1995. Two novel related yeast nucleoporins Nup170p and Nup157p: complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p. J. Cell Biol. 131(5): 1133–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, N. P. C., L. Huang, A. Burlingame and M. Rexach, 2001. Proteomic analysis of nucleoporin interacting proteins. J. Biol. Chem. 276(31): 29268–29274. [DOI] [PubMed] [Google Scholar]
- Amberg, D. C., A. L. Goldstein and C. N. Cole, 1992. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 6(7): 1173–1189. [DOI] [PubMed] [Google Scholar]
- Boeke, J. D., J. Trueheart, G. Natsoulis and G. R. Fink, 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154: 164–175. [DOI] [PubMed] [Google Scholar]
- Bogerd, A. M., J. A. Hoffman, D. C. Amberg, G. R. Fink and L. I. Davis, 1994. nup1 mutants exhibit pleiotropic defects in nuclear pore complex function. J. Cell Biol. 127(2): 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, J. W., K. D. Belanger, M. I. Sannella and L. I. Davis, 1999. The yeast nucleoporin Nup2p is involved in nuclear export of importin alpha/Srp1p. J. Biol. Chem. 274(45): 32360–32367. [DOI] [PubMed] [Google Scholar]
- Burda, P., C. A. Jakob, J. Beinhauer, J. H. Hegemann and M. Aebi, 1999. Ordered assembly of the asymmetrically branched lipid-linked oligosaccharide in the endoplasmic reticulum is ensured by the substrate specificity of the individual glycosyltransferases. Glycobiology 9(6): 617–625. [DOI] [PubMed] [Google Scholar]
- Chial, H. J., M. P. Rout, T. H. Giddings and M. Winey, 1998. Saccharomyces cerevisiae Ndc1p is a shared component of nuclear pore complexes and spindle pole bodies. J. Cell Biol. 143(7): 1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, M., N. Feinstein, K. L. Wilson and Y. Gruenbaum, 2003. Nuclear pore protein gp210 is essential for viability in HeLa cells and Caenorhabditis elegans. Mol. Biol. Cell 14(10): 4230–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, L. I., and G. Blobel, 1987. Nuclear pore complex contains a family of glycoproteins that includes p62: glycosylation through a previously unidentified cellular pathway. Proc. Natl. Acad. Sci. USA 84(21): 7552–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, L. I., and G. R. Fink, 1990. The NUP1 gene encodes an essential component of the yeast nuclear pore complex. Cell 61(6): 965–978. [DOI] [PubMed] [Google Scholar]
- Del Priore, V., C. Heath, C. Snay, A. MacMillan, L. Gorsch et al., 1997. A structure/function analysis of Rat7p/Nup159p, an essential nucleoporin of Saccharomyces cerevisiae. J. Cell Sci. 110(23): 2987–2999. [DOI] [PubMed] [Google Scholar]
- Drummond, S. P., and K. L. Wilson, 2002. Interference with the cytoplasmic tail of gp210 disrupts “close apposition” of nuclear membranes and blocks nuclear pore dilation. J. Cell Biol. 158(1): 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrog, B., J. Koser and U. Aebi, 2004. The nuclear pore complex: A jack of all trades? Trends Biochem. Sci. 29(4): 175–182. [DOI] [PubMed] [Google Scholar]
- Finlay, D. R., and D. J. Forbes, 1990. Reconstitution of biochemically altered nuclear pores: transport can be eliminated and restored. Cell 60(1): 17–29. [DOI] [PubMed] [Google Scholar]
- Finlay, D. R., D. D. Newmeyer, T. M. Price and D. J. Forbes, 1987. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J. Cell Biol. 104(2): 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, T., K. Strasser, A. Racz, S. Rodriguez-Navarro, M. Oppizzi et al., 2002. The mRNA export machinery requires the novel Sac3p-Thp1p complex to dock at the nucleoplasmic entrance of the nuclear pores. EMBO J. 21(21): 5843–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floer, M., and G. Blobel, 1999. Putative reaction intermediates in Crm1-mediated nuclear protein export. J. Biol. Chem. 274(23): 16279–16286. [DOI] [PubMed] [Google Scholar]
- Gomez-Ospina, N., G. Morgan, T. H. Giddings, Jr., B. Kosova, E. Hurt et al., 2000. Yeast nuclear pore complex assembly defects determined by nuclear envelope reconstruction. J. Struct. Biol. 132(1): 1–5. [DOI] [PubMed] [Google Scholar]
- Gorsch, L. C., T. C. Dockendorff and C. N. Cole, 1995. A conditional allele of the novel repeat-containing yeast nucleoporin RAT7/NUP159 causes both rapid cessation of mRNA export and reversible clustering of nuclear pore complexes. J. Cell Biol. 129(4): 939–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber, U. F., and L. Gerace, 1992. Nuclear protein import is inhibited by an antibody to a lumenal epitope of a nuclear pore complex glycoprotein. J. Cell Biol. 116(1): 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber, U. F., A. Senior and L. Gerace, 1990. A major glycoprotein of the nuclear pore complex is a membrane-spanning polypeptide with a large lumenal domain and a small cytoplasmic tail. EMBO J. 9(5): 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and G. R. Fink, 1991. Guide to Yeast Genetics and Molecular Biology. Academic Press, San Diego.
- Helenius, A., and M. Aebi, 2001. Intracellular functions of N-linked glycans. Science 291(5512): 2364–2369. [DOI] [PubMed] [Google Scholar]
- Hurwitz, M. E., and G. Blobel, 1995. NUP82 is an essential yeast nucleoporin required for poly(A)+ RNA export. J. Cell Biol. 130(6): 1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz, M. E., C. Strambio-de-Castillia and G. Blobel, 1998. Two yeast nuclear pore complex proteins involved in mRNA export form a cytoplasmically oriented subcomplex. Proc. Natl. Acad. Sci. USA 95(19): 11241–11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna, M. A., J. G. Petranka, J. L. Reilly and L. I. Davis, 1996. Yeast N1e3p/Nup170p is required for normal stoichiometry of FG nucleoporins within the nuclear pore complex. Mol. Cell. Biol. 16(5): 2025–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer, D. M., C. Strambio-de-Castillia, G. Blobel and M. P. Rout, 1995. The essential yeast nucleoporin NUP159 is located on the cytoplasmic side of the nuclear pore complex and serves in karyopherin-mediated binding of transport substrate. J. Biol. Chem. 270(32): 19017–19021. [DOI] [PubMed] [Google Scholar]
- Lau, C. K., T. H. Giddings, Jr. and M. Winey, 2004. A novel allele of Saccharomyces cerevisiae NDC1 reveals a potential role for the spindle pole body component Ndc1p in nuclear pore assembly. Eukaryot. Cell 3(2): 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb, J. D., L. I. Davis and G. R. Fink, 1993. NUP2, a novel yeast nucleoporin, has functional overlap with other proteins of the nuclear pore complex. Mol. Biol. Cell 4(2): 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier, M., A. M. White, J. Sheraton, T. di Paolo, J. Treadwell et al., 1997. Large scale identification of genes involved in cell surface biosynthesis and architecture in Saccharomyces cerevisiae. Genetics 147: 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney, W. C., and D. Duksin, 1979. Biological activities of the two major components of tunicamycin. J. Biol. Chem. 254(14): 6572–6576. [PubMed] [Google Scholar]
- Marelli, M., C. P. Lusk, H. Chan, J. D. Aitchison and R. W. Wozniak, 2001. A link between the synthesis of nucleoporins and the biogenesis of the nuclear envelope. J. Cell Biol. 153(4): 709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. W., M. R. Caracciolo, W. K. Berlin and J. A. Hanover, 1999. Phosphorylation and glycosylation of nucleoporins. Arch. Biochem. Biophys. 367(1): 51–60. [DOI] [PubMed] [Google Scholar]
- Nanduri, J., S. Mitra, C. Andrei, Y. Liu, Y. Yu et al., 1999. An unexpected link between the secretory path and the organization of the nucleus. J. Biol. Chem. 274(47): 33785–33789. [DOI] [PubMed] [Google Scholar]
- Pyhtila, B., and M. Rexach, 2003. A gradient of affinity for the karyopherin Kap95p along the yeast nuclear pore complex. J. Biol. Chem. 278(43): 42699–42709. [DOI] [PubMed] [Google Scholar]
- Ross-Macdonald, P., A. Sheehan, G. S. Roeder and M. Snyder, 1997. A multipurpose transposon system for analyzing protein production, localization, and function in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94(1): 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout, M. P., J. D. Aitchison, A. Suprapto, K. Hjertaas, Y. Zhao et al., 2000. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J. Cell Biol. 148(4): 635–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout, M. P., J. D. Aitchison, M. O. Magnasco and B. T. Chait, 2003. Virtual gating and nuclear transport: the hole picture. Trends Cell Biol. 13(12): 622–628. [DOI] [PubMed] [Google Scholar]
- Ryan, K. J., and S. R. Wente, 2002. Isolation and characterization of new Saccharomyces cerevisiae mutants perturbed in nuclear pore complex assembly. BMC Genet. 3(1): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, K. J., J. M. McCaffery and S. R. Wente, 2003. The Ran GTPase cycle is required for yeast nuclear pore complex assembly. J. Cell Biol. 160(7): 1041–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaich, N. L., and E. C. Hurt, 1995. Analysis of nucleocytoplasmic transport and nuclear envelope structure in yeast disrupted for the gene encoding the nuclear pore protein Nup1p. Eur. J. Cell. Biol. 67(1): 8–14. [PubMed] [Google Scholar]
- Shulga, N., P. Roberts, Z. Gu, L. Spitz, M. M. Tabb et al., 1996. In vivo nuclear transport kinetics in Saccharomyces cerevisiae: a role for heat shock protein 70 during targeting and translocation. J. Cell Biol. 135(2): 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulga, N., P. James, E. A. Craig and D. S. Goldfarb, 1999. A nuclear export signal prevents Saccharomyces cerevisiae Hsp70 Ssb1p from stimulating nuclear localization signal-directed nuclear transport. J. Biol. Chem. 274(23): 16501–16507. [DOI] [PubMed] [Google Scholar]
- Shulga, N., N. Mosammaparast, R. Wozniak and D. S. Goldfarb, 2000. Yeast nucleoporins involved in passive nuclear envelope permeability. J. Cell Biol. 149(5): 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn, L. A., T. Shen, N. Shulga, D. S. Goldfarb and S. R. Wente, 2004. Minimal nuclear pore complexes define FG repeat domains essential for transport. Nat. Cell Biol. 6(3): 197–206. [DOI] [PubMed] [Google Scholar]
- Stutz, F., M. Neville and M. Rosbash, 1995. Identification of a novel nuclear pore-associated protein as a functional target of the HIV-1 Rev protein in yeast. Cell 82(3): 495–506. [DOI] [PubMed] [Google Scholar]
- Suntharalingam, M., and S. R. Wente, 2003. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev. Cell 4(6): 775–789. [DOI] [PubMed] [Google Scholar]
- Tcheperegine, S. E., M. Marelli and R. W. Wozniak, 1999. Topology and functional domains of the yeast pore membrane protein Pom152p. J. Biol. Chem. 274(8): 5252–5258. [DOI] [PubMed] [Google Scholar]
- Travers, K. J., C. K. Patil, L. Wodicka, D. J. Lockhart, J. S. Weissman et al., 2000. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101(3): 249–258. [DOI] [PubMed] [Google Scholar]
- Wente, S. R., M. P. Rout and G. Blobel, 1992. A new family of yeast nuclear pore complex proteins. J. Cell Biol. 119(4): 705–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, R. A., and R. D. Gietz, 2001. High-efficiency transformation of plasmid DNA into yeast. Methods Mol. Biol. 177: 85–97. [DOI] [PubMed] [Google Scholar]
- Wozniak, R. W., E. Bartnik and G. Blobel, 1989. Primary structure analysis of an integral membrane glycoprotein of the nuclear pore. J. Cell Biol. 108(6): 2083–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak, R. W., G. Blobel and M. P. Rout, 1994. POM152 is an integral protein of the pore membrane domain of the yeast nuclear envelope. J. Cell Biol. 125(1): 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel, U., V. Doye, H. Tekotte, R. Wepf, P. Grandi et al., 1996. Nic96p is required for nuclear pore formation and functionally interacts with a novel nucleoporin, Nup188p. J. Cell Biol. 133(6): 1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]