Abstract
Recent studies demonstrated that pattern formation in plants involves regulation of transcription factor families by microRNAs (miRNAs). To explore the potency, autonomy, target range, and functional conservation of miRNA genes, a systematic comparison between plants ectopically expressing pre-miRNAs and plants with corresponding multiple mutant combinations of target genes was performed. We show that regulated expression of several Arabidopsis thaliana pre-miRNA genes induced a range of phenotypic alterations, the most extreme ones being a phenocopy of combined loss of their predicted target genes. This result indicates quantitative regulation by miRNA as a potential source for diversity in developmental outcomes. Remarkably, custom-made, synthetic miRNAs vectored by endogenous pre-miRNA backbones also produced phenocopies of multiple mutant combinations of genes that are not naturally regulated by miRNA. Arabidopsis-based endogenous and synthetic pre-miRNAs were also processed effectively in tomato (Solanum lycopersicum) and tobacco (Nicotiana tabacum). Synthetic miR-ARF targeting Auxin Response Factors 2, 3, and 4 induced dramatic transformations of abaxial tissues into adaxial ones in all three species, which could not cross graft joints. Likewise, organ-specific expression of miR165b that coregulates the PHABULOSA-like adaxial identity genes induced localized abaxial transformations. Thus, miRNAs provide a flexible, quantitative, and autonomous platform that can be employed for regulated expression of multiple related genes in diverse species.
INTRODUCTION
A major component of pattern formation in plants involves complex interplay between transcription factors (TFs) expressed in precise temporal and spatial domains and modifiers that act to maintain and refine their expression boundaries. TFs are usually expressed at low levels and guide the activity of many downstream effectors. Evolutionary expansion of TF families in plants (Riechmann et al., 2000) has meant that functional redundancy is a common theme in plant genomes. The majority of recently identified plant microRNAs (miRNAs) impose sequence-based simultaneous downregulation of developmentally important TFs (Llave et al., 2002a; Rhoades et al., 2002). Indirect evidence suggests that plant miRNAs have the potential to act efficiently to eliminate or clear cells of their target gene activities (Bartel, 2004). For instance, a large reduction in the activity of miRNA-regulated targets, either at the RNA or protein level, is evident upon ectopic miRNA expression (Aukerman and Sakai, 2003; Palatnik et al., 2003; Achard et al., 2004; Chen, 2004; Li et al., 2005; Schwab et al., 2005). Complementing these observations, strong dominant phenotypes are induced by target genes upon release of miRNA-guided regulation by mutations in their miRNA binding sites, and misexpression of miRNA-resistant targets with mutations at the miRNA binding site results in much stronger phenotypes than those obtained with native transcripts (reviewed in Chen, 2005). However, there is also evidence for quantitative action of plant miRNAs in quenching, as opposed to clearing, of the target gene activity. Thus, miRNA-resistant mutants are inherited in an incompletely dominant manner and are still subject to some miRNA-directed cleavage (Tang et al., 2003; Mallory et al., 2004a), and mutations in miRNA genes can result in increased levels of target gene expression (Baker et al., 2005).
Despite molecular indications that miRNAs can be very efficient, direct phenotypic evidence derived from comparing multiple mutant combinations with the effects of ectopic expression of the corresponding miRNA is limited. Unlike animal miRNAs, which simultaneously negatively regulate dozens of targets (Lim et al., 2005), plant miRNAs appear to have more limited target sets (Schwab et al., 2005), which are usually multiple members of the same gene family or even members of a single monophyletic clade of a larger family (Bartel and Bartel, 2003). These qualities can be used to facilitate the comparison of multiple mutant combinations with the effects of ectopic expression of the corresponding miRNA. In the absence of such a comparison, the prevailing model for high specificity and potency of plants miRNAs has yet to be verified. For example, in one case, ectopic miR164 induced cotyledon fusion and meristem arrest, mimicking the double mutant phenotype of two out of the five NAC domain genes targeted by this miRNA (Aida et al., 1997; Jones-Rhoades and Bartel, 2004; Laufs et al., 2004; Mallory et al., 2004b; Baker et al., 2005). However, overexpression of the miR165/6 failed to mimic the seedling arrest and production of a cylindrical monocot-like radial shoot observed in multiple mutants of the five miR165/6 PHABULOSA (PHB)-like targets (Emery et al., 2003; Li et al., 2005; Prigge et al., 2005; Williams et al., 2005a). The failure to recapitulate the phenotype in this latter case may reflect the inability of strong 35S:miR165/6 embryos to survive after transformation. Such a proposal could be confirmed by more precise viability-independent expression of the miR165/6.
Deciphering the role of TFs and their miRNA regulators in pattern formation can benefit greatly from spatial and temporal manipulation of their activities. This way, early deleterious effects can be bypassed and the autonomy of specific miRNA-induced perturbations examined. Such tissue-specific silencing using RNA interference (RNAi) has been successfully applied in plants (Watson et al., 2005), but RNAi has also been observed to induce systemic spread of gene silencing (reviewed in Voinnet, 2005). Notably, the common complexes involved in miRNA and short interfering RNA (siRNA) biogenesis and processing raises the possibility of a systemic component to miRNA-mediated regulation. In support of such a role, different miRNAs have been found in conducting phloem sap, a possible conduit for their systemic spread (Yoo et al., 2004). However, while unequivocal proof for long distance translocation of silencing signals was perceived through grafting experiments between silenced and nonsilenced transgenic tobacco (Palauqui et al., 1997), no such evidence has been provided for miRNA signals. Moreover, in mature Arabidopsis thaliana tissues, there is a strict overlap between pre miR171 promoter and miR171 activity monitored by a sensor construct, suggesting that this miRNA maintains strict spatial autonomy (Parizotto et al., 2004).
Many plant miRNAs appear to have a long coevolutionary history with their targets, extending back to moss and lycopods (Floyd and Bowman, 2004; Axtell and Bartel, 2005). The pairing structure of the miRNA and nearly perfect complementary miRNA* can be highly conserved. Though sequence conservation also occurs outside the domain of the miRNA and its complement, it is the general predicted structure of the pre-miRNA foldback, rather than its sequence per se, that is conserved between the distantly related Arabidopsis and rice (Oryza sativa) (Reinhart et al., 2002). This raises the question whether pre-miRNAs retain functional conservation between distant species. Such conservation would involve efficient miRNA biogenesis, including correct spatial recognition and processing of the miRNA/miRNA* from the pre-miRNA foldback structure (reviewed in Chen, 2005). Likewise, the coevolutionary history of miRNA and their targets also raises the question of whether unique characteristics define particular mRNAs as targets. Evidence from metazoans suggests that this is not the case, as the miRNA sequence and its complement in the foldback structure can be substituted and target novel transcripts (Dickins et al., 2005). Similarly, analysis in Arabidopsis demonstrated that the miRNA and its complement miRNA* domains of the pre miR171 backbone could be substituted to produce a novel miRNA that successfully targeted green fluorescent protein (GFP) (Parizotto et al., 2004). However, the capability and efficacy of the manipulated pre-miRNA in targeting multiple endogenous genes not normally targeted by miRNA regulation has yet to be examined.
In order to explore the extent and autonomy of downregulation that can be induced by miRNAs, we have chosen an experimental platform that uses sets of target genes for which complete conventional mutants are also available. Furthermore, miRNA ectopic expression has been brought under the control of in vivo constitutive or tissue-specific ectopic expression of pre-miRNAs guided by RNA Pol II promoters. This system enabled us to demonstrate that miRNAs have the potential to impose full or partial phenocopies of multiple mutants in several independent assays. These observations enabled us to expand the range of miRNA control by custom-designed pre-miRNAs that can stimulate phenocopy of mutations in genes not naturally regulated in this manner. Endogenous and synthetic Arabidopsis pre-miRNAs were functionally conserved in tomato (Solanum lycopersicum) and tobacco (Nicotiana tabacum), and tissue-specific miRNA misexpression produced phenotypes that are limited to the area of expression. Hence, these data suggest that miRNA activities are quantitative and, at least for distances greater than few cells, do not act outside of their domain of expression.
RESULTS
Precise Ectopic Expression of Endogenous miRNA Can Phenocopy Multiple Mutant Combinations
Plant miRNAs, such as miR164, have been ectopically expressed using the 35S promoter directly or through chemical induction driving the stem foldback that constitutes the pre-miRNA with various 5′ and 3′ endogenous additions (Figure 1A; Guo et al., 2005). We wished to examine the ability of precise spatial expression of miRNA activity to effectively monitor miRNA potential. Transactivation of protein-encoding genes using the LhG4-OP system has been effective in driving strong, specific expression and obviating early deleterious effects of gene overexpression (Moore et al., 1998). To assay the capacity of this system to express pre-miRNAs, genomic fragments containing the pre-miRNA foldback flanked by short 5′ and 3′ pre-miRNA sequences were cloned behind an OP array followed by a TATA box (see Supplemental Table 1 online). In the case of OP:miR164b, the resulting T2 lines (see Methods for selection and scoring of transgenic lines; summarized in Table 1) were transactivated using the PHB:LhG4 promoter, which is expressed in the developing cotyledon primordia and throughout the apical meristem (Figure 1C) to provide PHB≫miR164b F1s (Figure 1E; ≫ denotes transactivation). Expression of miR164 under this promoter mimicked the fused cotyledon and meristem arrest phenotype of the cup-shaped cotyledon1 (cuc1) and cuc2 double mutant, two of miR164's six targets (Figure 1D). These observations indicate that expression of pre miR164b using the transactivation system can efficiently deplete cells of target gene activities.
Figure 1.
miRNAs Can Quantitatively Regulate Multiple Transcripts Simultaneously and Phenocopy Their Combined Loss of Function.
(A) A scheme of an endogenous pre-miRNA. The red and blue fragments will be cleaved by DICER-LIKE1 (DCL1) to generate the miRNA and miRNA*, respectively.
(B) A 10-d-old wild-type seedling.
(C) The promoter of PHB drives GFP expression throughout the shoot apex in wild-type heart-stage embryos.
(D) Arabidopsis cuc1 cuc2 double mutant seedlings.
(E) F1 seedlings of OP:miR164b transactivated by PHB:LhG4.
(F) A monocot-like phv phb rev triple mutant seedling.
(G) A monocot-like PHB≫miR165b seedling of comparable age.
(H) Whole shoot and flower (inset) of 35S:miR167a plant next to same age wild type display identical alterations found in arf6 arf8 double mutants (cf. with Nagpal et al., 2005).
(I) Scanning electron micrograph of wild-type flowering apex.
(J) A cross section through AP1≫HP-GFP flowering apex with expression throughout emerging flower meristems.
(K) Wild-type flower.
(L) Fused sepals and absent petals in AP1≫miR164b flower.
(M) Flowering apex and filamentous flowers (inset) of strong AP1≫miR165b plant.
(N) Normal sepals, radial petals, distorted stamens, and multiple carpels in a weak AP1≫miR165b flower.
(O) Sequence alignment of the wild type and phv-1d mutant with corresponding miR165b and miR165bm6.
(P) Seedling expressing ANT≫miR165b#4 results in radialized cotyledons and aborted meristem.
(Q) and (R) Adaxial surface of wild-type (Q) and ANT≫miR165bm6 (R) leaves. Note the adaxial outgrowths of the transgenic leaf (arrow).
(S) Normal sepals, distorted stamens, and multiple carpels in a strong ANT≫miR165bm6 flower.
FM, flower meristem; IM, inflorescence meristem; P, petal. Bars = 3 mm in (B), (F), and (G) and 20 μm in (C), (I), and (J).
Table 1.
Endogenous and Synthetic miRNAs Examined in This Study
| miRNA and Its Pre-miRNA Backbone
|
Transgenes and Recipient Speciesa
|
Target Gene Function and Loss-of-Function Mutant Description
|
Frequency and Range of Phenotypic Responses in Independent Lines
|
|||
|---|---|---|---|---|---|---|
| Target Genes | Strong | Intermediate | Weak | |||
| miR164/pre miR164b | ArabidopsisOP:miR164bb | NAC domain TFs CUC1, CUC2, NAC1, At5g07680, At5g61430, At5g39610 | Embryonic meristem establishment, organ separation, and lateral root outgrowth (Figure 1D) | 10 | 0 | 0 |
| tomato OP:miR164bc | Tomato NACs | 2 | 8 | 1 | ||
| miR165/pre miR165b | ArabidopsisOP:miR165bb* | PHB-like class III HD-ZIP TFs PHV, CNA, REV, ATHB8, PHB | Meristem establishment and maintenance, adaxial differentiation of lateral organs, and vasculature patterning (Figure 1F) | 23 | 8 | 2 |
| tomato OP:miR165bc | Tomato PHB-like | 1 | 3 | 0 | ||
| miR165/pre miR165a | ArabidopsisOP:miR165ab* | As for miR165b | As for miR165b | 17 | 2 | 2 |
| miR166/pre miR166g | ArabidopsisOP:miR166gb* | As for miR165b | As for miR165b | 16 | 3 | 3 |
| miR165m6/pre miR165b | ArabidopsisOP:miR165m6c | As for miR165b but with reduced homology toits targets | As for miR165b | 1 | 1 | 0 |
| miR167/pre miR167a | Arabidopsis35S:miR167ab | ARF TFs ARF6, ARF8 | Promotion of flowering and JA-mediated flower organ maturation (Nagpal et al., 2005) | 8 | 10 | 2 |
| miR-ARF/pre miR164b | Arabidopsis35S:miR-ARFb | ARF TFs ARF2, ARF3/ETT, ARF4 | Abaxial differentiation of lateral organs, vascular development, flowering, and cell growth (Figure 2D) | 9 | 8 | 3 |
| tomato 35S:miR-ARFc | 3 | 4 | 0 | |||
| tobacco 35S:miR-ARFc | 2 | 6 | 0 | |||
| miR-NGA/pre miR164a | Arabidopsis35S:miR-NGAab | B3 domain TFs NGA1, NGA2, NGA3, NGA4 | Lateral organ growth (Figures 3E and 3H) | 5 | 9 | 6 |
| OP:miR-NGAab | 2 | 6 | 2 | |||
| tomato 35S:miR-NGAc | Weak homology with NGA-like/BI934304 and BI934637 | 0 | 5 | 4 | ||
| miR-NGA/pre miR164b | Arabidopsis35S:miR-NGAbb | As for miR-NGAa | As for miR-NGAa | 7 | 9 | 4 |
Frequent silencing was observed in subsequent generations for the lines marked with asterisks.
The 10OP:miRNA lines were examined upon transactivation with a promoter LhG4 driver. Combinations with specific promoters are described in the text. The 35S:miRNA lines were scored directly as T1s and showed consistent phenotypes upon cross with the wild type.
Phenotype relative to loss-of-function phenocopy (close resemblance is strong).
Phenotype scoring is relative to other transgenic lines carrying the same construct because corresponding mutants are not available.
To extend these observations to other Arabidopsis miRNAs, we expressed OP:miR165b using the promoter of one of its target genes, the PHB:LhG4 driver. miR165/6 target the five PHB-like genes that redundantly promote meristem establishment and maintenance as well as differentiation of lateral organs and the vasculature. phb phavoluta (phv) revoluta (rev) triple mutants or loss of all of the five PHB-like genes result in seedling arrest after the production of a cylindrical monocot-like radial shoot in which the apical meristem activity is abolished (Figure 1F; Emery et al., 2003; Prigge et al., 2005). However, previous overexpression studies with 35S:miR165 resulted in variable seedling phenotypes in which the most extreme plants had small leaves with some polarity defects (Li et al., 2005). By contrast, PHB≫miR165b plants gave rise to radial seedlings, phenocopying the multiple mutant combination (Figure 1G). These results illustrate that specific miRNA expression can abolish target gene activity and demonstrate the potential potency of precise pre-miRNA misexpression as a vehicle for simultaneous downregulation of multiple members of the same gene family.
35S-Driven miRNAs Can Faithfully Mimic Loss-of-Function Phenotypes
It is likely that the inefficiency of 35S:miR165a in producing a phenocopy of the PHB-like genes reduction is due to their spatial expression in early stage embryos and their importance in embryogenesis. Auxin Response Factor6 (ARF6) and ARF8 are the only predicted targets of miR167, and arf6 arf8 double mutants are viable, late flowering, have dark green leaves, and exhibit unexpanded 2nd and 3rd whorl floral organs (Nagpal et al., 2005). To assay whether the 35S promoter can effectively drive a miRNA-mediated reduction in these genes, we assayed 35S:pre miR167a transformants. Out of 20 independent T1 plants, eight had a similar phenotype to that reported for arf6 arf8 double mutants both vegetatively and in flowers, while the remainder had a range of weaker phenotypes (Figure 1H, Table 1). No additional features than described for the double mutants were noticed. Thus, depending on the miRNA and its targets, elevated ectopic miRNA expression by the constitutive 35S promoter can specifically reduce multiple target gene activities to levels that parallel that of multiple loss-of-function mutants.
Precise Expression of miRNAs Can Reveal Novel Mutation Patterns
Embryonic expression of either miR164 or miR165 resulted in a seedling phenocopy of multiple mutants in the corresponding target genes. While cuc1 cuc2 seedlings can be rescued using tissue culture, phb phv rev or plants mutant for all five PHB-like mutant genes do not develop beyond the seedling stage. Because the CUC-like and PHB-like genes are active throughout plant development, the use of tissue-specific miRNA expression could reveal functions of these genes later in plant development. To examine the utility of this approach, transactivation of selected strong OP:miR164b and OP:miR165b lines was performed using the promoter of the flower meristem gene APETALA1 (AP1).
As shown in Figures 1I and 1J, the expression mediated by AP1 promoter initiates transcript accumulation throughout young floral meristems. In AP1≫miR164b plants, the sepals are completely fused, petals are absent, and stamens exhibit fusion to each other and the gynoecium (cf. Figures 1L and 1K). This phenotype is similar, albeit more severe than that observed in cuc1 cuc2 flowers generated through tissue culture (Aida et al., 1997). In AP1≫miR165b plants, only radial filamentous structures were observed in the place of flowers, consistent with the miR165 effectively eliminating flower meristem function (Figure 1M). These findings are consistent with the observed seedling phenotypes, indicating that the PHB-like genes are essential for embryo and flower meristem maintenance. These observations demonstrate that the transactivation system can be effectively used in conjunction with miRNA-mediated loss of gene activity in specific cells at any stage of the plant's life cycle.
Low Levels of miRNA Expression or High Levels of Inefficient miRNA Suggest That Plant miRNAs Can Act in a Quantitative Fashion
Tissue-specific transactivation of pre-miRNAs identified lines producing a loss-of-function phenocopy but also uncovered OP:miRNA lines that induced mild phenotypes (Table 1). For example, when a weak OP:miR165b line was transactivated with the AP1:LhG4 promoter, the growth of the petals and stamens was markedly affected and additional carpels were formed (Figure 1N). Associations between phenotype strength and transcription levels have been shown previously for ectopic miR164 and miR166g expression (Laufs et al., 2004; Williams et al., 2005a). Likewise, assaying both the weak and strong OP:miR165b lines with the same promoters revealed consistent phenotype strengths restricted to the promoter's expression domain. These observations imply that when expressed in a discrete group of cells, miR165 can act in a quantitative fashion.
We hypothesized that quantitative action of miRNA may also be achieved by the generation of miRNA with lower homology. This was based on the observation that mRNA of the dominant phv-1d miRNA-resistant mutation was still cleaved (our unpublished data; Tang et al., 2003). The phv-1d molecular lesion is a G-to-A transition in the miR165/6 target region opposite position 6 from the 5′ end of the miR165/6 (Figure 1O). We created a theoretically less-efficient version of miR165b. Thus, miR165bm6 (C-to-U substitution in position 6 of the miR165b) mimics the corresponding position of the phv-1d mutation in the miRNA rather than in the target. The efficacy of the modified miRNA was demonstrated by its ability to restore wild-type morphology to the phv-1d mutant plants (see Supplemental Figure 1 online). The AINTEGUMENTA:LhG4 (ANT) promoter is expressed in the primordia of all above-ground organs. ANT≫miR165b plants exhibited a strong phenotype of radialized cotyledons and an aborted apical meristem (Figure 1P). By contrast, ANT≫miR165m6 plants had a weak, abaxialized phenotype, consistent with inefficient miR165bm6 action. The adaxial surface of the leaves developed localized outgrowths (no effect was observed on the abaxial leaf surface), while in the flowers, the stamens locules were reversed and the gynoecium, like that of weak AP1≫miR165b lines, had additional carpels (Figures 1Q to 1S). A similar floral phenotype was observed of AP1≫ miR165m6 plants (see Supplemental Figure 1 online). The mild abaxialized phenotype of OP:miR165bm6 plants and that of the weak OP:miR165b lines argues that miRNAs can act in a quantitative fashion depending on the pairing quality and quantity of the miRNA with respect to its target mRNAs.
General Strategy for Developing Synthetic miRNA on the Backbone of Native miRNA
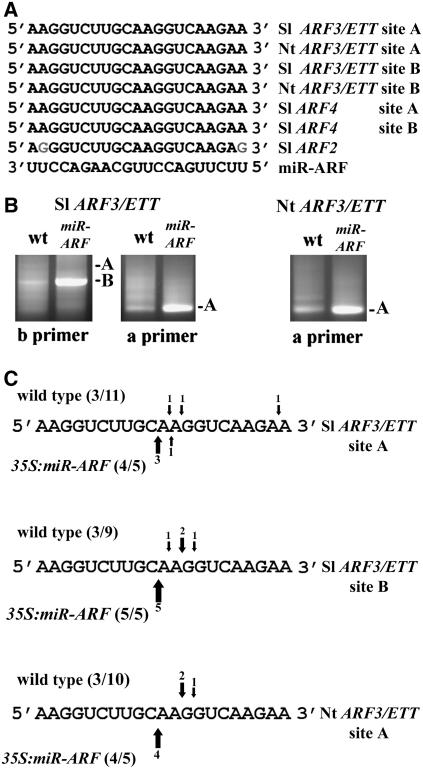
Ectopic expression of endogenous pre-miRNAs allowed controlled downregulation of multiple coregulated miRNA targets. This prompted us to explore the possibility to design synthetic miRNA that will target genes not normally regulated by miRNA. We used the pre miR164b backbone as a template to approach these questions based on the consistency and efficacy of miR164b in the overexpression analysis. In addition, this backbone contains a bulge at position 4 of the conceptualized Dicer-generated miRNA/miRNA* duplex in the desired miRNA strand (marked red in Figure 2B). Asymmetric instability in this hybrid is believed to help differentiate between the miRNA and miRNA* in the RNA-induced silencing complex (RISC) (Schwarz et al., 2003), thus theoretically helping define the miRNA (upper strand in pre-miRNA, Figure 2B) irrespective of the sequence introduced. As initial gene targets for synthetic miRNA regulation, we selected the abaxial promoting ARF3 and ARF4 along with ARF2, for which respective mutations have been described (Li et al., 2004; Pekker et al., 2005; Okushima et al., 2005b; Schruff et al., 2006). Importantly, these ARFs contain common, conserved sequences, which are the basis for negative coregulation by evolutionary conserved transacting siRNAs (ta-siRNAs), siR2141 and siR2142 (Allen et al., 2005; Williams et al., 2005b). The target sequences of these ta-siRNAs (two sites in ARF3/ETTIN [ETT] and ARF4 and one in ARF2) are conserved in Arabidopsis, tomato, and monocotyledonous plants and suggest that ARF2 may have overlapping functions with ARF3/ETT and ARF4 (Figure 2A; Pekker et al., 2005; Williams et al., 2005b). Exploiting this, we designed a miRNA that should directly target these three ARFs and ensured that it began with U, like most plant miRNAs. As shown in the bottom line of Figure 2B, mismatches were introduced into the miRNA complementary sequence to mimic the predicted stem of the miR164b precursor, assuming that bulges in the miR164 backbone contain essential recognition and processing information. The miR-ARF2/3/4 (hereafter referred to as miR-ARF) was integrated into the pre miR164b backbone by direct gene synthesis (Figure 2B, right panel), and the resulting pre miR-ARF was cloned behind the 35S or OP promoter.
Figure 2.
Custom-Designed Synthetic miRNAs Efficiently Regulate ta-siRNA Targets.
(A) Sequence alignment of the ta-siRNA binding sites in Arabidopsis ARF2, ARF3, and ARF4, the endogenous ta-siRNAs, and a designed miR-ARF sequence with better homology to all target sites. The A and B sites are designated according to Allen et al. (2005). Mismatches are marked red and G-U wobbles cyan.
(B) Predicted folding and dicing of the pre miR164b backbone before (left) and after (right) replacement of miR164 with the miR-ARF sequence.
(C) Abaxial side of wild-type (a), ett-1 arf4-1 (b), and 35S:miR-ARF (c) leaves.
(D) Bolting shoot of ett-1 arf4-1 plant.
(E) Bolting shoot of 35S:miR-ARF plant.
(F) to (H) Flowers of wild-type (F), ett-1 arf4-1 (G), and 35S:miR-ARF plants (H). Note the gradual increase in the number of sepals, stamen radialization, and decrease in petal width. sp, sepal; p, petal; st, stamen.
(I) Detection of miR-ARF and miR164 in wild-type and 35S:miR-ARF plants by RNA gel blot analysis. Both miRNAs are the same ∼21 nucleotides.
(J) Reduced levels of full-length transcripts of the three ARF genes in 35S:miR-ARF plants.
(K) A scheme of ARF4 cDNA with primers used for RLM-RACE detection. Gel images showing RLM-RACE–detected wild-type and 35S:miR-ARF (miR-ARF) cleavage products at sites A and B detected using either primer a or b, where A is the expected gel position for a product cleaved at site A, and B is the expected gel position of product cleaved at site B. In 35S:miR-ARF, amplification products are more prevalent relative to the wild type. In addition, amplification of a product cleaved at site A could only be obtained with the a primer (gels for PCR products of ARF2 and ARF3 cleavage analysis are shown in Supplemental Figure 2 online).
(L) Summary of cleavage analysis by direct sequencing of RLM-RACE products (see Supplemental Figure 2 for details) and product cloning of the three ARF genes. Cleavage analysis for 35S:miR-ARF plants is shown, and the dispersed cleavage products of the wild type are shown in Supplemental Figure 2 online and in Allen et al. (2005). DS, direct sequencing.
Synthetic miRNA Efficiently Regulates ARF2, 3, and 4, Which Are Naturally Regulated by Transacting siRNAs
Arabidopsis ett/arf3 plants are characterized by a loss of abaxial identity in the gynoecium, while in ett/arf3 arf4 double mutants, all lateral organs exhibit reduced abaxial identity and outgrowths on the abaxial surface of their leaves (Figure 2C; Pekker et al., 2005). Triple ett/arf3 arf4 arf2 mutants are unavailable due to the tight genetic linkage between arf4 and arf2 (Okushima et al., 2005a). Strikingly, all 35S:miR-ARF Arabidopsis transformants exhibited phenotypes consistent with an effective targeting of the three ARF genes. Plants with a strong phenotype resembled ett/arf3 arf4 double mutants but had more outgrowths on the abaxial leaf surface (Figures 2C to 2E, Table 1) as well as an increased floral organ number and frequently radialized stamens (Figures 2F to 2H). Similar to arf2 single mutants, these 35S:miR-ARF plants were also late flowering (Ellis et al., 2005; Okushima et al., 2005b).
A Synthetic miRNA Born on a Stem-Loop Precursor Efficiently Guides RISC Complexes
We isolated RNA from plants of a 35S:miR-ARF line with a strong phenotype and verified by RNA gel blot analysis that miR-ARF was efficiently processed from its precursor by identifying its 21-nucleotide miRNA, which was comparable in size with the endogenous miR164 (Figure 2I). After processing, miRNAs enter the RISC complex and guide cleavage of their target transcripts, inducing their degradation and/or interference with translation (Llave et al., 2002b; Aukerman and Sakai, 2003; Chen, 2004). To examine the mode of action of the synthetic miRNA, we assayed the relative abundance of the full-length ARF transcripts using RNA gel blot analysis. For all three, a significant but incomplete reduction in RNA levels was observed in 35S:pre miR-ARF Arabidopsis plants (Figure 2J).
Cleavage of the three ARF genes by the synthetic miR-ARF was investigated using 5′ RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) analysis (Kasschau et al., 2003). Endogenous ta-siRNA–directed cleavage (siR2141 and siR2142) of ARF transcripts has been shown in Arabidopsis (Allen et al., 2005; Williams et al., 2005b). However, a much greater abundance of cleaved products of all three ARF genes was observed in 35S:miR-ARF plants than in wild-type Arabidopsis (Figure 2K; see Supplemental Figure 2 online). In wild-type plants, we could detect products at relatively low amounts from the ta-siRNA–mediated cleavage at both ARF3/ARF4 A and ARF3/ARF4 B sites using primers downstream of the B site (primer b, Figure 2K). For 35S:miR-ARF plants, we managed to detect only B-site cleavage products with primer B, but using a different primer (primer a between the A and B sites for ARF4), we amplified abundant products from cleavage at site A (Figure 2K). In the 35S:miR-ARF plants, sequence analysis demonstrated that cleavage in the target mRNA occurred consistently between nucleotides 10 and 11 at miR-ARF complementary sites (marked in Figure 2L), while much more dispersed cleavage products were amplified from the wild type (see Supplemental Figure 2 online; Allen et al., 2005). We therefore conclude that miRNAs generated by a stem-loop precursor guide the RISC complexes more specifically than ta-siRNA–mediated cleavage.
Synthetic miRNA Efficiently Regulates the NGATHA Multigene Family Not Normally Associated with RISC-Mediated Control
To develop the use of synthetic miRNA beyond gene families that are normally regulated by various RISC-based small RNA regulation, we next targeted the NGATHA (NGA) genes for which there is no evidence of this form of regulation.
The NGA clade is composed of four genes (NGA1-4); all are closely related to the RAV-like proteins in the structure of the B3-DNA binding domain but do not have an AP2 domain (Figure 3A). Like ett mutations, mutants in nga1 phenotypically enhance the kanadi1 mutant phenotype in the gynoecium (Bowman et al., 2002; nga1 was renamed from howard). We identified NGA1 and found that all four NGA genes redundantly regulate lateral organ growth (as will be described elsewhere). However, only minor morphological alterations are apparent in any of the single gene mutants. A single consensus stretch of 21 nucleotides within the B3 domain was identified that could constitute a common synthetic miRNA target site in the four NGA-like genes of Arabidopsis (Figure 3B). This 21-nucleotide sequence fulfilled the available criteria of allowable mismatches in number and position as well as low free energy characterizing endogenous plant miRNAs (Figure 3B; Allen et al., 2005; Schwab et al., 2005). The 21-nucleotide specificity was confirmed in the genome via BLAST search and was modified to begin with a U like most plant miRNAs. The designed 21 nucleotides were introduced into the miR164a and miR164b backbones to generate miR-NGAa and miR-NGAb, respectively (Figure 3C).
Figure 3.
Custom-Designed Synthetic miRNA Efficiently and Specifically Codownregulate Non-Native Small RNA Targets.
(A) A phylogenetic tree of the NGA-like proteins and their closest Arabidopsis homologs. Tree was constructed with the ∼120 amino acids that constitute the B3 domain as shown in Supplemental Figure 3 online. ETT was included as an outgroup, and numbers represent bootstrap percentage from 1000 trials.
(B) A general scheme of NGA1/2/3/4 transcripts, outlining the position of a consensus sequence aligned. A synthetic miRNA has 0 to 2 mismatches with all four, and its conceptual dicing from pre miR164b backbone is illustrated. The arrow above pileup denotes cleavage point as described below.
(C) Predicted folding and dicing of the pre miR164a backbone before (left) and after (right) replacement of miR164 with the miR-NGA sequence.
(D) to (F) Young seedling of wild-type (D), nga1-1 nga2-1 nga3-1 nga4-1 quadruple mutant (E), and 35S:miR-NGAa (F) plants. Note the angular leaf blade of the mutants compared with the round leaf blade of the wild type. cd, cotyledons.
(G) to (I) Inflorescence and pre-anthesis flower (insets) of wild-type (G), nga1-1 nga2-1 nga3-1 nga4-1 quadruple mutant (H), and 35S:miR-NGAa (I) plants. Note the broad yellowish petals and the protruding distal portion of the unfused gynoecium (gy).
(J) Cotransactivation of NGA1 and miR-NGAa by CAB3 promoter eliminates dwarfism induced by ectopic NGA1 with the same promoter line (inset).
(K) Detection of miR-NGA in wild-type and 35S:miR-NGAa plants by RNA gel blot analysis.
(L) RLM-RACE detection of cleaved products of the four NGA-like transcripts in 35S:miR-NGAa (miR-NGA) plants but not wild-type plants.
(M) RNA gel blot analysis reveals differential reduction in RNA levels of NGA1 and NGA3 in wild-type and 35S:miR-NGAa plants.
nga1 nga2 nga3 nga4 quadruple mutant plants have shorter and wider leaves than the wild type, sepals and petals are broad, and petals are slightly green-yellow. Style development is severely impaired, with disruptions in the coordinated growth that normally seal the two-carpel gynoecium. Consequently, the gynoecium remains distally unfused, with distinctive projections emanating from the top of the valves (cf. Figures 3E and 3H with 3D and 3G). Remarkably, plants of 35S:miR-NGAa and 35S:miR-NGAb showed phenotypic alterations approaching that of the nga1 nga2 nga3 nga4 quadruple mutant plants (Figures 3F to 3I, Table 1). These results show that miRNA-dependent control can be extended to other multigene families.
NGA RNA Analysis Detects Cleavage Products and Differential Reduction of NGA RNA
We asked whether the molecular processing of NGA RNA by miR-NGA is similar to RISC-mediated processes. An RNA gel blot from RNA extracted from plants of a line with a strong phenotype confirmed the presence of the 21-nucleotide synthetic miR-NGA, and RLM-RACE analysis identified miRNA-directed cleavage products (Figures 3K and 3L). No evidence of NGA-like cleavage products was observed in RNA from wild-type inflorescences. Notably, the predominant cleavage position as determined by direct sequencing of the RLM-RACE products was identical in all four NGA genes and was comparable to a common cleavage site for endogenous miRNAs (including that of miR-164b) occurring between positions opposing 10 and 11 5′ of the miR-NGA (as marked in Figure 3B). However, though RNA levels of NGA1 were reduced in 35S:miR-NGAa plant inflorescences, very mild reduction in the levels of NGA3 was detected (Figure 3M). This could be due to attenuation of protein translation or a transcriptional autofeedback loop.
We next asked whether miR-NGA could ameliorate the phenotype produced by overexpression of the NGA1 gene. The CAB3:LhG4 (chlorophyll a/b binding promoter) line drives expression throughout photosynthetic tissues, and CAB3≫NGA1 plants are dwarfed with small leaves (Figure 3J). However, in plants where both NGA1 and miR-NGA were cotransactivated by CAB3:LhG4 (CAB3≫NGA1 and miR-NGAa, Figure 3J), plant stature and leaf growth resembled that of CAB3≫miR-NGAa plants (a near wild-type phenotype; data not shown), indicating that the levels of NGA protein from NGA gene overexpression were efficiently reduced by miR-NGA.
Thus, custom-designed miRNAs that follow a few basic rules of pairing are capable of simultaneously and efficiently downregulating multiple transcripts that are not naturally regulated by small RNAs and induce morphological changes that match computer predictions for target specificity.
Arabidopsis Pre-miRNAs Can Effectively Regulate Cross-Species Targets
To investigate the functional conservation of Arabidopsis pre-miRNA processing in distantly related species, we first introduced OP:miR164b into tomato, a distantly related plant species that shows conserved miR164 targets (N. Ori, personal communication). The progenies of two selected 35S≫miR164b lines had fused cotyledons (Figure 4A), a similar phenotype to the petunia (Petunia hybrida) no apical meristem mutant (Souer et al., 1996). Thus, misexpression of pre miR164b resulted in uniform, heritable, and stable lines capable of simultaneous downregulation of multiple members of the same gene family both in Arabidopsis and in tomato. We also expressed the OP:miR165b in tomato plants whose homologous target genes are also conserved (our analysis) to explore whether this functional conservation of pre-miRNAs in other species is a general phenomenon. The At FILAMENTOUS FLOWER (FIL) promoter drives expression throughout tomato leaf primordia before becoming abaxially restricted (Lifschitz et al., 2006). FIL≫miR165b plants had abaxialized, filamentous leaves consistent with miR165b function in downregulating the adaxial-promoting PHB-like genes in tomato (Figures 4B and 4C). The abaxial nature of the filamentous leaves was evident by the prevalence of long linear trichomes and the absence of short globular ones, a typical composition of abaxial leaf epidermis (Reinhardt et al., 2005).
Figure 4.
Arabidopsis Pre-miRNA Backbones Induce Homologous Mutant Phenotypes Heterologously.
(A) Wild-type and F1 tomato seedlings of OP:miR164b transactivated by 35S:LhG4. The top and bottom insets are close-ups of the upper portion of the seedling.
(B) and (C) Upper part of wild-type (B) and FIL≫miR165b (C) tomato shoots. Leaves in (C) are short and radial, and floral organs are narrow. l, leaf; f, flower; rl, radialized leaf.
(D) Abaxial side of wild-type and 35S:miR-ARF leaves. A close-up of 35S:miR-ARF leaf at the right illustrates the abaxial-specific outgrowths found along the veins.
(E) to (H) Flowers ([E] and [F]) and carpels ([G] and [H]) of wild-type ([E] and [G]) and 35S:miR-ARF ([F] and [H]) tomato plants. Note the thin sepals and petals, the short style, and the thickened green stigma of the transformant. st, style; sg, stigma.
(I) to (K) Comparison of leaves (I), flowers (J), and carpels (K) of wild-type (left) and 35S:miR-ARF tobacco plants. Gradual effects from weak to strong (right) are notable in independent T1 plants. As in tomato, abaxial leaf outgrowths are evident along the veins. Corolla outgrowths are external (abaxial) only.
(L) and (M) Gynoecium of wild-type (L) and ett-1 (M) Arabidopsis plants. As in tomato and tobacco, stigmatic tissue in the mutant is expanded basally while style length is reduced.
Subsequently, we tested whether the synthetic pre miR-ARF would also function in other species. 35S:miR-ARF tobacco and tomato transformants exhibited phenotypes consistent with the specific transformations of abaxial cell types into adaxial ones observed in 35S:miR-ARF Arabidopsis plants. The leaves of 35S:miR-ARF tomato plants had small, misshapen, up-curled leaflets that were darker green and developed outgrowths on their abaxial surface (Figure 4D). The inflorescence structure approximated that of the wild type, but in the flowers, the number of sepals was increased, sepals and petals were narrower and shorter, and white rather than green carpels were topped by thick, green style/stigmatic tissue (Figures 4E to 4H). Such redistribution of gynoecium cell types is similar to single mutations in ett/arf3 in Arabidopsis, where style tissues are expanded basally (Figures 4L and 4M).
Similarly, 35S:miR-ARF tobacco lines had small, up-curling leaves that, in more severe lines, developed outgrowths on the abaxial leaf surface (Figure 4I). Flowering was delayed, and when it finally occurred in severe lines, the corolla remained largely green. Strikingly, very pronounced abaxial outgrowths developed around the entire corolla circumference, mostly in its distal third (Figure 4J). However, even in the most severe lines, the adaxial (inner) side of the corolla surface was unchanged. As in tomato, the gynoecia had basally expanded green stigma (Figure 4K). These results confirm and extend previous suggestions on the central role of the three ARFs in promotion of abaxial identity in all lateral organs in Arabidopsis and allow expansion of this role to the remotely related Solanaceae.
To associate the 35S:miR-ARF phenotype of tomato and tobacco with miR-ARF activity, 5′ RLM-RACE was used. As with Arabidopsis, tomato ARF3/ETT has two presumptive ta-siRNA target sites and, thus, two miR-ARF target sites (Figure 5A). In 35S:miR-ARF tomato plants, we detected much higher levels of cleavage at both A and B sites than in wild-type tomato plants (Figure 5B). Notably, the predominant cleavage position within the target site was shifted one nucleotide 3′ relative to that observed in Arabidopsis (Figure 5C). These observations suggest that the pre miR-ARF is functionally conserved in tomato. In tobacco, we only tested miR-ARF activity at the A site of the ARF3/ETT homolog by cleavage analysis. However, the level of cleaved transcript detected was again greater than that observed in wild-type tobacco plants and occurred predominantly at the same position within the target domain that was observed in tomato (Figures 5B and 5C).
Figure 5.
Induction of Solanaceae Target Cleavage by Arabidopsis-Based Synthetic Pre-miRNAs.
(A) Sequence alignment of tomato ARF2/3/4, tobacco ETT/ARF3, and miR-ARF. The nucleotides not in gray are predicted wobbles.
(B) Gel images showing RLM-RACE detection of tomato and tobacco ARF3 cleavage products from wild-type and 35S:miR-ARF (miR-ARF) plants. Primer position follows the ARF4 design in Figure 2K. Cleavage products at sites A and B were detected using either primer a or b, where A is the expected gel position for a product cleaved at site A, and B is the expected gel position of product cleaved at site B.
(C) Cleavage point mapping of tomato and tobacco ARF3. Arrows mark cleavage sites in sequenced, cloned products, where arrow size corresponds with frequency of clones obtained (also given). Arrows above each sequence are for clones obtained from the wild type, and those below are from 35S:miR-ARF plants. The number of clones matching the predicted target region out of total number of sequenced clones is shown in parentheses.
Use of the 35S:miR-NGAa construct in tomato resulted in a fruit phenotype, and miR-NGA–mediated cleavage in the tomato NGA-like transcripts (NGA-like/BI934304 and BI934637) was detected, albeit at low levels (see Supplemental Figure 3 online). In this case, it is likely that mismatches in the critical 5′ region of the miR-NGA relative to the target mRNAs obviate efficient miRNA regulation.
Cell-Autonomous Effects of miRNA Expression
It has been suggested that miRNA activities might not be cell-autonomous (e.g., short-range movement of miR166 was suggested to account for restriction of the PHB-like activities from the abaxial side of emerging organ primordial) (Juarez et al., 2004; Kidner and Martienssen, 2004). The potency of miRNA target regulation and its efficacy across species allowed examination of cell autonomy by the different criteria of tissue specificity and gross systemic spread. To examine the autonomy of miRNA action, we specifically expressed pre miR166g, pre miR165a, or pre miR165b in the 2nd and 3rd whorl floral organ primordia of Arabidopsis using an AP3 driver line (AP3:LhG4; Figure 6A, Table 1). As expected, in AP3≫miR165/6 plants, petals and stamens became completely radialized, a typical morphology for organs lacking abaxial/adaxial asymmetry (Figure 6C). In the upper part of the stamens, guard cells that are typically restricted to the abaxial connective tissue were present all around (Figure 6D). In contrast with the 2nd and 3rd whorl organs, normal gynoecia were formed (Figure 6C), suggesting autonomous restriction imposed on miR165/6 activity or, alternatively, insensitivity of the gynoecium. Thus, we expressed pre miR165b by the gynoecium-specific promoter of CRABS CLAW (CRC) that drives expression throughout the gynoecium valve anlagen before becoming abaxially restricted (Figure 6B). In CRC≫miR165b plants, a thin gynoecium was formed, without adaxial placenta and ovules, while the stamens and petals were unaffected (Figure 6E). Thus, expression of pre miR165/6 in cells of organ primordia bordering other primordia provided no evidence for expanded miRNA activity.
Figure 6.
miRNA Activities Are Spatially Restricted and Do Not Cross Graft Joints.
(A) Transverse section of an Arabidopsis flower showing promoter AP3-mediated expression of GFP limited to petals and stamen.
(B) Longitudinal section of same age Arabidopsis flower showing that promoter CRC-mediated expression of GFP is limited to carpels.
(C) Flower of AP3≫miR165b plant with radial petals and stamens but normal gynoecium.
(D) Close-up of radial stamens with normally abaxial-restricted guard cells scattered all around (arrows).
(E) Flower of CRC≫miR165b plant with thin gynoecium and normal stamens.
(F) A graft of 35S:miR-ARF on wild-type tomato. Picture was taken 4 months after grafting and after two rounds of defoliation (arrows) of wild-type leaves. Newly initiating shoots remain normal. White box shows graft union.
(G) to (I) A graft of the wild type on 35S:miR-ARF tobacco (G). Here, too, defoliation (arrows) of wild-type leaves did not stimulate miRNA-derived phenotype on wild-type shoot. The white box shows the graft union. Upon flowering, wild-type acceptor shoots were normal (H) even though 35S:miR-ARF donor flowers are highly distinct (I).
sp, sepal; p, petal; st, stamen; gy, gynoecium; rp, radial petal; rst, radial stamen. Bars = 50 μm in (A) and (B) and 100 μm in (C) and (D).
The striking phenotype of tomato and tobacco plants expressing 35S:pre miR-ARF provided an opportunity to phenotypically assay for systemic translocation of this miRNA. In six out of six reciprocal grafts between strong 35S:miR-ARF and wild-type tomato, both rootstock and scion maintained autonomous morphology over 6 months (Figure 6F). Likewise, the dramatic morphological effects of ectopic miR-ARF expression did not cross graft barriers in two out of two reciprocal tobacco grafts between 35S:miR-ARF and the wild type, even though graft union supported viable scions for over 6 months (Figures 6G to 6I). As long-range movement often follows source to sink flow, we repeatedly defoliated the wild-type shoots after complete graft union was obtained in both tomato and tobacco (arrows in Figures 6F and 6G). After 2 months of such repeated defoliation, all new emerging leaves and subsequently floral primordia had a wild-type appearance. Thus, using morphological criteria, no support for short or long-range translocation of miRNA-induced alterations was observed.
DISCUSSION
Pattern formation of plant organs often involves regulation of TF families by miRNAs. In this study, we have systematically demonstrated that miRNA can simultaneously quench the activity of their multiple predicted TF targets to levels matched by conventional loss-of-function mutations. However, no morphological alterations beyond the computational predictions for target specificity were encountered, suggesting very limited and precise targeting. The efficient silencing potential was not restricted to genes naturally regulated by small RNAs, and Arabidopsis pre-miRNA backbones were efficiently processed to induce homologous loss-of-function phenotypes in distantly related tomato and tobacco. Finally, we have demonstrated that miRNA activities can be quantitative, do not cross graft barriers, and at least for short distances, exert autonomous effects.
Gene Silencing with miRNAs
Functional overlap (redundancy) presents a difficult logistic obstacle for in vivo characterization of gene function in general and more so in plants that in many cases have undergone multiple cycles of polyploidization and subsequent selective gene loss (Moore and Purugganan, 2005). Here, we show that miRNAs can potentially abolish simultaneously the activities of all of their targets to levels matched by conventional loss-of-function mutations. Endogenous pre-miRNA–encoding genes of Arabidopsis could be modified to specifically target (sequence allowing) selected gene families. The range of phenotypic perturbations, even at the high levels of ectopic expression, did not exceed that of corresponding multiple mutant combinations in the predicted target genes, suggesting that off-targets are not significantly affected. It was also possible to reduce the complementarity of the miRNA to its targets and to produce weaker phenotypes. The specific sequence requirements for recognition of targets by their corresponding miRNA were formulated earlier (Allen et al., 2005; Schwab et al., 2005). Following these basic rules, we could custom design synthetic miRNAs that were as efficient as endogenous ones in simultaneous downregulation of multiple targets, and all are members of the same gene family. Using such sequence-specific design, it should be possible to specifically target unique isoforms generated via alternative splicing or target multiple homologs ordered in tandem. Such application might be highly significant for plant genomes; it is estimated that 29% of the rice genes are organized in clusters of gene families (International Rice Genome Sequencing Project, 2005) and are not amenable for functional analysis via classical genetic tools.
Most of the miRNAs and gene family targets found in plants have a long coevolutionary history. Nearly all highly conserved miRNAs and corresponding gene target sites are identical in Arabidopsis and rice, and some are also shared with nonvascular plants (Floyd and Bowman, 2004; Axtell and Bartel, 2005). Comparison of the pre-miRNA genes between Arabidopsis and other species shows some conservation beyond the miRNA and its complement to provide similarity in predicted folding (Reinhart et al., 2002; Floyd and Bowman, 2004). Using phenotypic and molecular assays, we found that pre-miRNAs were processed efficiently when the miRNA sequence and its complement were substituted in Arabidopsis and that these synthetic Arabidopsis miRNAs and their native counterparts functioned in distantly related Solanaceae species. Recent work in mammal cells suggests that the miRNA RISC loading complex processes the pre-miRNA directly, unwinding and loading the mature miRNA to Argonaute2 of the RISC (Maniataki and Morelatos, 2005). Thus, basic, conserved structural elements of the pre-miRNA secondary structure may be sufficient for miRNA RISC loading complex recognition and processing, and these are maintained in the manipulations we performed.
Endogenous plant miRNAs and the synthetic miRNAs we tested have a high degree of homology to their targets and mediated gene suppression primarily through cleavage. In metazoans, miRNA-mediated cleavage also occurs where the miRNA has a high degree of homology with the coding region of its target (Yekta et al., 2004). Using appropriate computational analyses, this effective and stable mechanism for gene targeting can be broadly applied in all organisms with miRNA machinery.
RNAi versus miRNA Silencing
Long hairpin RNAi constructs provide perfectly matched double-stranded RNA strands cleaved to short siRNAs, while miRNAs are processed from precursors containing mismatches and bulges. We assume that it is those mismatches that provide structural information for Dicer activities guiding precise cleavage of the same 21 nucleotides. As such information is missing in the perfect match stems, a mixture of mers is produced unless phased first by precise guiding cleavage (Allen et al., 2005). Moreover, even though a single miRNA molecule is generated per one pre-miRNA, efficient downregulation of multiple targets with matching sequence homology can be achieved. This result is in contrast with the long stem-loop RNAi vectors or cosuppression lines where downregulation of a specific transcript only is commonly evident. For example, Chuang and Meyerowitz (2000) demonstrated specific downregulation of AP1 using RNAi. However, despite the sequence being used having long stretches of perfect homologies with the homologous CAULIFLOWER (CAL) gene, no ap1 cal-like plants were observed. Likewise, RNAi-targeted suppression of ARF2 in Arabidopsis (Li et al., 2004) and ARF4 in tomato (Jones et al., 2002) resulted in a specific single mutant phenotype, in contrast with our results. Keeping in mind that double-stranded RNA is also amplified and dependent on the activities of RNA-dependent RNA polymerase (RdRp), stoichiometric effects of miRNAs appear to be much more potent (Sijen et al., 2001).
Quantitative versus Qualitative Regulation by miRNAs
Most plant miRNAs target TFs that play critical roles in morphogenesis. In animals, recent studies suggest that miRNAs and their targets are expressed in adjacent, largely nonoverlapping domains through transcriptional control (Stark et al., 2005). Under this scenario, miRNAs function to confer robustness on gene expression boundaries. In plants, the evidence of miRNA function paints a more complex picture. It has been proposed that miRNAs act to clear cells of their target gene activities as supported by the strong developmental phenotypes of dominant miRNA-resistant mutations (Rhoades et al., 2002). Such a mode of action is attractive for modeling formation and stabilization of sharp boundaries between adjacent expression domains. However, observations of miR165/6 and miR164 expression and action relative to their respective PHB-like and CUC-like targets suggest a more quantitative role for the miRNA through dampening down target gene expression levels in cells coexpressing both (McConnell et al., 2001; Bao et al., 2004; Baker et al., 2005; Li et al., 2005; Williams et al., 2005a). In both cases of miRNA/target pairs, increased target levels were observed when either the target was resistant to its miRNA or when endogenous miRNAs were reduced by mutation. Since zonation of the plant apical meristem into central and peripheral domains is an ongoing, dynamic process, the slow gradual restrictions of expression domains of both CUC-like and PHB-like multiple family members likely reflects a more fluid regulation of these genes by their corresponding miRNAs. Similarly, during leaf morphogenesis, cell position rather than lineage is important until relatively late in leaf development (reviewed in Scheres, 2001). Therefore, proper leaf morphogenesis involves a continual interplay between adaxial-promoting PHB-like and abaxial-promoting ARF2/3/4 TFs, each class with supporting small RNA regulation (miR165/6 and siR2141/2, respectively) to reinforce cell identity. In such a scenario, the ta-siRNAs and miR165/6 may be coexpressed with, and fine-tune, target gene expression as part of the ongoing process of acquiring and translating positional information.
Our experiments with endogenous pre miR165b, pre miR164b, and pre miR167a overexpression indicate that plant miRNAs have the potential to act in a quantitative fashion. High and/or precise pre-miRNA expression of these miRNAs mimicked multiple mutants of the target genes, demonstrating that these plant miRNAs have the potential to clear cells of their target gene activities. However, weak overexpression elicited mild phenotypes only, presumably by reducing and not eliminating target gene function. Thus, it appears that a broad range of plant miRNAs can act quantitatively, depending on expression levels. The weak abaxialized phenotype obtained from strong specific overexpression of the miR165bm6 with reduced homology to its PHB-like gene transcripts (Figure 1; see Supplemental Figure 1 online) also suggests that miRNA target mismatches are a potential source for inefficiency in miRNA action. A striking feature of endogenous plant miRNAs is the evolutionary conservation of miRNA target mismatches, even between the critical 5′ region of the miRNA and its target (Axtell and Bartel, 2005). The synthetic miR-ARF and miR-NGA we assayed had perfect homology to target regions in ETT/ARF4 and NGA2, respectively. From the simple perspective of thermodynamic rules of pairing, this should maximize the miR-ARF and miR-NGA in terms of negative regulation of these targets. While we cannot say whether perfect miRNA target homology is the most efficient form of these or any other miRNAs, our results suggest that perfect-match miRNAs are as efficient as native ones. If a perfect target match is the most efficient form of a miRNA, the evolutionary conservation of mismatches suggests a selection for miRNA inefficiency. One possibility is that an inefficient miRNA, combined with variable levels of pre-miRNA transcription, contributes to a wider range of target gene outputs, which can be translated into a morphogenetic gradient. Thus, it can be speculated that while plant miRNAs have the capacity to clear cells of target gene activities, they act endogenously to both clear cells of their targets and to dampen their expression, depending on the miRNA and developmental context.
Local versus Systemic Regulation by miRNA
The quantitative nature of miRNA regulation and the transient nature of the pattern formation process do not support a role for long-distance miRNA transport. In agreement, we could not find any evidence for translocation of miR-ARF effects across graft barriers in tomato and tobacco. In Arabidopsis, we also failed to find phenotypic evidence of miRI65a/b or miR166g moving small cellular distances in the developing flower. Still, a context-dependent short-range movement (one to two cells) of selected miRNAs in particular domains cannot be ruled out, a feature common to other macromolecules shown to traffic from cell to cell (Gallagher et al., 2004; Kurata et al., 2005). DCL4 activities are required for cell-to-cell spread of transgene-born silencing signals (Dunoyer et al., 2005), suggesting that siRNAs do provide the spreading sequence-based information agent. Significantly, mutations in DCL4 or in components of the RdRP machinery that abolish systemic silencing spread do not affect patterning. Thus, miRNAs and siRNAs might not serve as equal substrates for trafficking. siRNAs contrast with miRNAs in having a perfect match with their target mRNA. In this respect, one possible role for mismatches between miRNAs and their targets is that they act as a means to avoid the miRNA acting as a template for the RdRP silencing machinery. The miR-ARF, which has a perfect match to ARF3/ETT and ARF4 of tomato and ARF3/ETT of tobacco, failed to elicit a systemic silencing response, suggesting that the miRNA/target mismatches may not function in this process. Thus, regulated expression of synthetic miRNA can allow for targeting of multiple transcripts for simultaneous sector analysis and for bypassing early lethality associated with multiple mutant combinations.
METHODS
Plant Material
The Arabidopsis thaliana plants described are all in the Landsberg erecta background. Plants were grown under 18 h of cool white fluorescent light at 20°C. M82 tomato (Solanum lycopersicum) and Samsun tobacco (Nicotiana tabacum) plants were grown in greenhouse conditions with temperatures ranging between 18 and 25°C. The nga1 mutation was identified in a gymnos kanadi background (Bowman et al., 2002). Map-based identification of At2g46870 as the gene mutant in nga1 will be described elsewhere. nga1 has a premature stop codon at Gln-203. nga2-1 (At3g61970) (a kind gift from Venkatesan Sundaresan) contains a Ds insertion at +142 from the first Met codon within the B3 domain-encoding region. nga3-1 (At1g01030) has a T-DNA insertion (Garlic/Sail_232_E10) at position +238 from the first Met codon, likewise within the B3-encoding domain, and nga4-1 (At4g01500) (also a kind gift from Venkatesan Sundaresan) has a DS insertion +112 from the first Met codon, again within the B3 domain. nga1-1 nga2-1 nga3-1 nga4-1 quadruple mutant plants were produced by conventional breeding. In the F2, presumptive quadruple mutant plants with a distinct phenotype appeared at an approximate ratio of 1:256, and their genotype was verified using PCR (see Supplemental Table 3 online for primer details).
Grafting
We used a classic wedge-shaped/slit grafting technique with the site of union wrapped by Parafilm. Plants were kept for 2 to 3 d in the shade and for 7 d in 80% humidity provided by plastic bags. Success exceeds 90%.
Pre-miRNA Clones and Plant Transformation
For isolation of pre-miRNA–containing sequences, the genomic DNA flanking the predicted stem-loop of different, annotated pre-miRNAs was amplified using PCR (see supplemental tables online for primers and details). Details on the stem-loop and short 5′ and 3′ ends are summarized in Supplemental Table 1 online. The pre miR165bm6 and pre miR-NGAa synthetic genes were generated by assembly PCR, as detailed in the legend of Supplemental Table 2 online. The pre miR-ARF and pre miR-NGAb synthetic genes were synthesized by Epoch Biolabs and DNA 2.0, respectively. To produce pre-miRNA stem-loop representations, we used the Web-based mfold program (Zuker, 2003). After sequence verification, the pre-miRNA clones were cloned behind an OP array (10OP-TATA-BJ36) or 35S promoter (ART7) and transferred into the binary pMLBART (for Arabidopsis) or pART27 (for tomato and tobacco) vectors (Eshed et al., 2001). A 6-kb PHB promoter and a 6.1-kb At FIL promoter were PCR amplified with the primers in Supplemental Table 2 online. FIL, PHB, and 35S promoters were subcloned in front of LhG4 (Moore et al., 1998) and subsequently cloned into the pART27 or pMLBART binary vector. Other transactivation driver lines were described earlier (Pekker et al., 2005). Cotyledon transformation in tomato and leaf disc transformation in tobacco were performed according to McCormick (1991) and Horsch et al. (1985), respectively. Arabidopsis transgenic lines were generated by the floral dip method, and BASTA-resistant transformants were selected on soil.
Evaluation of Transgenic Lines
Two types of evaluation of transgenic lines were performed. Primary 35S:miRNA T1 lines were scored directly as having a strong, intermediate, or weak phenotype. Selected 2 to 10 lines (fertility allowing) were backcrossed (BC) with the wild type, and a minimum of 30 BC1 progeny were scored to assay phenotype severity and heritability. Unless otherwise mentioned, original phenotypes were faithfully transmitted, and only lines with a single T-DNA insert locus were used for molecular analyses. Alternatively, in order to screen for independent, primary 10OP:pre-miRNA transformants, 10 to 30 T1 plants were crossed to homozygous promoter:LhG4 lines, and a minimum of 30 promoter≫miRNA F1 plants per cross were scored. Each primary line was designated as strong, intermediate, or weak on the basis of the morphology of the F1 progenies. Only 10OP:pre-miRNA lines with single, unlinked T-DNA insert loci were used for further experiments. In all cases, unless otherwise mentioned, strong lines were used for morphological and molecular characterizations.
RNA Isolation and Analysis
Total RNA was extracted using TRI Reagent (Sigma-Aldrich) according to the manufacturer's instructions. High molecular weight RNA was normalized by spectrophotometry to 20 μg/lane. Radiolabeled probes for RNA gel blot analysis of mRNAs were made by random priming reactions. Probes consisted of the 3′ (to the stop codon) 1049 bp (ETT), 1497 bp (ARF4), 1459 bp (ARF2), 617 bp (NGA1), and 828 bp (NGA3) of these genes annotated cDNAs. Equivalent loading of samples was monitored by detection of 28S and 18S RNA in all gels prior to blot transfer.
Low molecular weight RNA was purified with an RNeasy plant mini kit (Qiagen), resolved on a 17% polyacrylamide-urea gel, transferred to a Zeta-Probe GT membrane (Bio-Rad), and probed with a 32P end-labeled oligonucleotide, complementary to the mature miRNAs (see Supplemental Table 4 online). Equivalent loading of samples was shown by detection of 5S RNA in all gels prior to blot transfer.
Isolation of Tomato and Tobacco cDNAs
The complete coding sequences of the ARF2, ARF3/ETT, and ARF4 genes of tomato were cloned using primers designed from the alignment of different tomato EST sequences in GenBank. A partial ARF3/ETT gene of N. tabacum was isolated using homology with the tomato ARF3/ETT gene (see supplemental tables online for primer details). Young leaves, apices, and flowers were harvested, and total mRNA was extracted from vegetative and reproductive shoot tips using Trizol reagent. cDNA template was synthesized from 1 μg of total RNA using Superscript II reverse transcriptase (Invitrogen) or PowerScript RT enzyme (Clontech).
RACE Analysis of Cleaved miRNA Target Genes
Cleavage sites in the miRNA target genes were mapped using RLM-RACE, a modified 5′ RACE procedure as described by Kasschau et al. (2003), using the GeneRacer (Invitrogen) protocol coupled with nested gene-specific primers ∼200 to 400 nucleotides downstream of the predicted miRNA target site (see Supplemental Table 5 online). The PCR products were purified and directly sequenced or cloned into pDrive (Qiagen) or pCRII (Sigma-Aldrich) and sequenced.
Microscopy and Confocal Imaging
Tissue preparation and histological analyses were performed according to Pekker et al. (2005). Scanning electron microscopy was performed using an XL30 ESEM FEG microscope (FEI). For confocal imaging, tissue was fixed in 2.5% paraformaldehyde overnight, osmotically adjusted, and frozen, and 20- to 45-μm sections were made with a Leica 2000 microtome. Fluorescence was observed by an Olympus CLSM500 microscope with an argon laser at 488 nm for excitation and 505 to 525 nm for GFP emission.
Accession Numbers
Tomato ARF sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers DQ340254 (Sl ETT), DQ340259 (Sl ARF4), and DQ340255 (Sl ARF2). Accession numbers for tobacco are DQ340258 (Nt ARF2), and DQ340256 and DQ340257 (Nt ARF3/ETT).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table 1. Sequence Parameters of Pre-miRNA.
Supplemental Table 2. Primers for PCR-Mediated Cloning.
Supplemental Table 3. Primers for PCR-Mediated Genotyping of NGATHA2-4 Insertion Alleles.
Supplemental Table 4. Oligonucleotides for miRNA Detection by RNA Gel Blot Analysis and Primers for RLM-RACE.
Supplemental Table 5. Gene-Specific Primers for RLM-RACE.
Supplemental Figure 1. Design of and Additional Phenotypes Induced by miR165bm6.
Supplemental Figure 2. Analysis of Cleavage Products of Arabidopsis ARF Genes.
Supplemental Figure 3. NGA-Like Gene Alignment and Cleavage Analysis in Tomato.
Supplementary Material
Acknowledgments
We thank Eugenia Klein and the electron microscopy facility for help with scanning electron microscopy, Raya Eilam for help with tissue preparation techniques, and Vladimir Kiss for assistance with confocal laser scanning microscopy. We also thank Venkatesan Sundaresan, Michael Lenhard, the SIGNAL collection of Syngenta Seeds, and the ABRC stock center for providing plasmids and plant material. We thank John Bowman, Robert Fluhr, Naomi Ori, and members of Y.E.'s lab for comments and discussions and Detlef Weigel for sharing unpublished results. This work was made possible with funding from Grants 3328-02 from the U.S.–Israel Binational Agricultural Research and Development Fund and 386-02 from the Israel Science Foundation. J.P.A. is an honorary research fellow of the School of Biological Sciences (Monash University). Y.E. is an incumbent of the Judith and Martin Freedman Career Development Chair.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Yuval Eshed (yuval.eshed@weizmann.ac.il).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.040725.
References
- Achard, P., Herr, A., Baulcombe, D.C., and Harberd, N.P. (2004). Modulation of floral development by a gibberellin-regulated microRNA. Development 131 3357–3365. [DOI] [PubMed] [Google Scholar]
- Aida, M., Ishida, T., Fukaki, H., Fujisawa, H., and Tasaka, M. (1997). Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. Plant Cell 9 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, E., Xie, Z., Gustafson, A.M., and Carrington, J.C. (2005). microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121 207–221. [DOI] [PubMed] [Google Scholar]
- Aukerman, M.J., and Sakai, H. (2003). Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15 2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell, M.J., and Bartel, D.P. (2005). Antiquity of microRNAs and their targets in land plants. Plant Cell 17 1658–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, C.C., Sieber, P., Wellmer, F., and Meyerowitz, E.M. (2005). The early extra petals1 mutant uncovers a role for microRNA miR164c in regulating petal number in Arabidopsis. Curr. Biol. 15 303–315. [DOI] [PubMed] [Google Scholar]
- Bao, N., Lye, K.W., and Barton, M.K. (2004). MicroRNA binding sites in Arabidopsis class III HD-ZIP mRNAs are required for methylation of the template chromosome. Dev. Cell 7 653–662. [DOI] [PubMed] [Google Scholar]
- Bartel, B., and Bartel, D.P. (2003). MicroRNAs: At the root of plant development? Plant Physiol. 132 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel, D.P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116 281–297. [DOI] [PubMed] [Google Scholar]
- Bowman, J.L., Eshed, Y., Baum, S., Emery, J.F., Floyd, S.K., Alvarez, J., Hawker, N.P., Lee, J.Y., Siegfried, K.R., Khodosh, R., Tatom-Jaurez, M., and Perea, J.V. (2002). The story of CRABS CLAW (or How we learned to love the mutagen). Flowering Newsletter 31 3–11. [Google Scholar]
- Chen, X. (2004). A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303 2022–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. (2005). MicroRNA biogenesis and function in plants. FEBS Lett. 579 5923–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang, C.F., and Meyerowitz, E.M. (2000). Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 97 4985–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickins, R.A., Hemann, M.T., Zilfou, J.T., Simpson, D.R., Ibarra, I., Hannon, G.J., and Lowe, S.W. (2005). Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet. 37 1289–1295. [DOI] [PubMed] [Google Scholar]
- Dunoyer, P., Himber, C., and Voinnet, O. (2005). DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat. Genet. 37 1356–1360. [DOI] [PubMed] [Google Scholar]
- Ellis, C.M., Nagpal, P., Young, J.C., Hagen, G., Guilfoyle, T.J., and Reed, J.W. (2005). AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 132 4563–4574. [DOI] [PubMed] [Google Scholar]
- Emery, J.F., Floyd, S.K., Alvarez, J., Eshed, Y., Hawker, N.P., Izhaki, A., Baum, S.F., and Bowman, J.L. (2003). Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 13 1768–1774. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., Baum, S.F., Perea, J.V., and Bowman, J.L. (2001). Establishment of polarity in lateral organs of plants. Curr. Biol. 11 1251–1260. [DOI] [PubMed] [Google Scholar]
- Floyd, S.K., and Bowman, J.L. (2004). Gene regulation: Ancient microRNA target sequences in plants. Nature 428 485–486. [DOI] [PubMed] [Google Scholar]
- Gallagher, K.L., Paquette, A.J., Nakajima, K., and Benfey, P.N. (2004). Mechanisms regulating SHORT-ROOT intercellular movement. Curr. Biol. 14 1847–1851. [DOI] [PubMed] [Google Scholar]
- Guo, H.S., Xie, Q., Fei, J.F., and Chua, N.H. (2005). MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell 17 1376–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch, R.B., Fry, J.E., Hoffmann, N.L., Eichholtz, D., Rogers, S.G., and Fraley, R.T. (1985). A simple and general method for transferring genes into plants. Science 227 1229–1231. [DOI] [PubMed] [Google Scholar]
- International Rice Genome Sequencing Project (2005). The map-based sequence of the rice genome. Nature 436 793–800. [DOI] [PubMed] [Google Scholar]
- Jones, B., Frasse, P., Olmos, E., Zegzouti, H., Li, Z.G., Latche, A., Pech, J.C., and Bouzayen, M. (2002). Down-regulation of DR12, an auxin-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J. 32 603–613. [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades, M.W., and Bartel, D.P. (2004). Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol. Cell 14 787–799. [DOI] [PubMed] [Google Scholar]
- Juarez, M.T., Kui, J.S., Thomas, J., Heller, B.A., and Timmermans, M.C. (2004). MicroRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428 84–88. [DOI] [PubMed] [Google Scholar]
- Kasschau, K.D., Xie, Z., Allen, E., Llave, C., Chapman, E.J., Krizan, K.A., and Carrington, J.C. (2003). P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA unction. Dev. Cell 4 205–217. [DOI] [PubMed] [Google Scholar]
- Kidner, C.A., and Martienssen, R.A. (2004). Spatially restricted microRNA directs leaf polarity through ARGONAUTE1. Nature 428 81–84. [DOI] [PubMed] [Google Scholar]
- Kurata, T., et al. (2005). Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development 132 5387–5398. [DOI] [PubMed] [Google Scholar]
- Laufs, P., Peaucelle, A., Morin, H., and Traas, J. (2004). MicroRNA regulation of the CUC genes is required for boundary size control in Arabidopsis meristems. Development 131 4311–4322. [DOI] [PubMed] [Google Scholar]
- Li, H., Johnson, P., Stepanova, A., Alonso, J.M., and Ecker, J.R. (2004). Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev. Cell 7 193–204. [DOI] [PubMed] [Google Scholar]
- Li, H., Xu, L., Wang, H., Yuan, Z., Cao, X., Yang, Z., Zhang, D., Xu, Y., and Huang, H. (2005). The putative RNA-dependent RNA polymerase RDR6 acts synergistically with ASYMMETRIC LEAVES1 and 2 to repress BREVIPEDICELLUS and microRNA165/166 in Arabidopsis leaf development. Plant Cell 17 2157–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz, E., Eviatar, T., Rozman, A., Shalit, A., Goldshmidt, A., Amsellem, Z., Alvarez, J.P., and Eshed, Y. (2006). The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- Lim, L.P., Lau, N.C., Garrett-Engele, P., Grimson, A., Schelter, J.M., Castle, J., Bartel, D.P., Linsley, P.S., and Johnson, J.M. (2005). Microarray analysis shows that some microRNAs down regulate large numbers of target mRNAs. Nature 433 769–773. [DOI] [PubMed] [Google Scholar]
- Llave, C., Kasschau, K.D., Rector, M.A., and Carrington, J.C. (2002. a). Endogenous and silencing-associated small RNAs in plants. Plant Cell 14 1605–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llave, C., Xie, Z., Kasschau, K.D., and Carrington, J.C. (2002. b). Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297 2053–2056. [DOI] [PubMed] [Google Scholar]
- Mallory, A.C., Dugas, D.V., Bartel, D.P., and Bartel, B. (2004. b). MicroRNA regulation of NAC-domain targets is required for proper formation and separation of adjacent embryonic, vegetative, and floral organs. Curr. Biol. 14 1035–1046. [DOI] [PubMed] [Google Scholar]
- Mallory, A.C., Reinhart, B.J., Jones-Rhoades, M.W., Tang, G., Zamore, P.D., Barton, M.K., and Bartel, D.P. (2004. a). MicroRNA control of PHABULOSA in leaf development: Importance of pairing to the microRNA 5′ region. EMBO J. 23 3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniataki, E., and Morelatos, Z. (2005). A human, ATP-independent, RISC assembly machine fueled by pre-miRNA. Genes Dev. 19 2979–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell, J.R., Emery, J., Eshed, Y., Bao, N., Bowman, J., and Barton, M.K. (2001). Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411 709–713. [DOI] [PubMed] [Google Scholar]
- McCormick, S. (1991). Transformation of tomato with Agrobacterium tumifaciens. In Plant Tissue Culture Manual, Vol. B6, K. Lindsey, ed (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 1–9.
- Moore, I., Galweiler, L., Grosskopf, D., Schell, J., and Klaus, P.A. (1998). Transcription activation system for regulated gene expression in transgenic plants. Proc. Natl. Acad. Sci. USA 95 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, R.C., and Purugganan, M.D. (2005). The evolutionary dynamics of plant duplicate genes. Curr. Opin. Plant Biol. 8 122–128. [DOI] [PubMed] [Google Scholar]
- Nagpal, P., Ellis, C.M., Weber, H., Ploense, S.E., Barkawi, L.S., Guilfoyle, T.J., Hagen, G., Alonso, J.M., Cohen, J.D., Farmer, E.E., Ecker, J.R., and Reed, J.W. (2005). Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 132 4107–4118. [DOI] [PubMed] [Google Scholar]
- Okushima, Y., Mitina, I., Quach, H.L., and Theologis, A. (2005. b). AUXIN RESPONSE FACTOR 2 (ARF2): A pleiotropic developmental regulator. Plant J. 43 29–46. [DOI] [PubMed] [Google Scholar]
- Okushima, Y., et al. (2005. a). Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: Unique and overlapping functions of ARF7 and ARF19. Plant Cell 17 444–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik, J.F., Allen, E., Wu, X., Schommer, C., Schwab, R., Carrington, J.C., and Weigel, D. (2003). Control of leaf morphogenesis by microRNAs. Nature 425 257–263. [DOI] [PubMed] [Google Scholar]
- Palauqui, J.C., Elmayan, T., Pollien, J.M., and Vaucheret, H. (1997). Systemic acquired silencing: Transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 16 4738–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizotto, E.A., Dunoyer, P., Rahm, N., Himber, C., and Voinnet, O. (2004). In vivo investigation of the transcription, processing, endonucleolytic activity, and functional relevance of the spatial distribution of a plant miRNA. Genes Dev. 18 2237–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekker, I., Alvarez, J.P., and Eshed, Y. (2005). Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell 17 2899–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge, M.J., Otsuga, D., Alonso, J.M., Ecker, J.R., Drews, G.N., and Clark, S.E. (2005). Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart, B.J., Weinstein, E.G., Rhoades, M.W., Bartel, B., and Bartel, D.P. (2002). MicroRNAs in plants. Genes Dev. 16 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, D., Frenz, M., Mandel, T., and Kuhlemeier, C. (2005). Microsurgical and laser ablation analysis of leaf positioning and dorsoventral patterning in tomato. Development 132 15–26. [DOI] [PubMed] [Google Scholar]
- Rhoades, M.W., Reinhart, B.J., Lim, L.P., Burge, C.B., Bartel, B., and Bartel, D.P. (2002). Prediction of plant microRNA targets. Cell 110 513–520. [DOI] [PubMed] [Google Scholar]
- Riechmann, J.L., et al. (2000). Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 290 2105–2110. [DOI] [PubMed] [Google Scholar]
- Scheres, B. (2001). Plant cell identity. The role of position and lineage. Plant Physiol. 125 112–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schruff, M.C., Spielman, M., Tiwari, S., Adams, S., Fenby, N., and Scott, R.J. (2006). The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 133 251–261. [DOI] [PubMed] [Google Scholar]
- Schwab, R., Palatnik, J.F., Riester, M., Schommer, C., Schmid, M., and Weigel, D. (2005). Specific effects of microRNAs on the plant transcriptome. Dev. Cell 8 517–527. [DOI] [PubMed] [Google Scholar]
- Schwarz, D.S., Hutvagner, G., Du, T., Xu, Z., Aronin, N., and Zamore, P.D. (2003). Asymmetry in the assembly of the RNAi enzyme complex. Cell 115 199–208. [DOI] [PubMed] [Google Scholar]
- Sijen, T., Fleenor, J., Simmer, F., Thijssen, K.L., Parrish, S., Timmons, L., Plasterk, R.H.A., and Fire, A. (2001). On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107 465–476. [DOI] [PubMed] [Google Scholar]
- Souer, E., van Houwelingen, A., Kloos, D., Mol, J., and Koes, R. (1996). The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 85 159–170. [DOI] [PubMed] [Google Scholar]
- Stark, A., Brennecke, J., Bushati, N., Russell, R.B., and Cohen, S.M. (2005). Animal microRNAs confer robustness to gene expression and have a significant impact on 3′UTR evolution. Cell 123 1133–1146. [DOI] [PubMed] [Google Scholar]
- Tang, G., Reinhart, B.J., Bartel, D.P., and Zamore, P.D. (2003). A biochemical framework for RNA silencing in plants. Genes Dev. 17 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet, O. (2005). Non-cell autonomous RNA silencing. FEBS Lett. 579 5858–5871. [DOI] [PubMed] [Google Scholar]
- Watson, J.M., Fusaro, A.F., Wang, M., and Waterhouse, P.M. (2005). RNA silencing platforms in plants. FEBS Lett. 579 5982–5987. [DOI] [PubMed] [Google Scholar]
- Williams, L., Carles, C.C., Osmont, K.S., and Fletcher, J.C. (2005. b). A database analysis method identifies an endogenous trans-acting short-interfering RNA that targets the Arabidopsis ARF2, ARF3, and ARF4 genes. Proc. Natl. Acad. Sci. USA 102 9703–9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, L., Grigg, S.P., Xie, M., Christensen, S., and Fletcher, J.C. (2005. a). Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development 132 3657–3668. [DOI] [PubMed] [Google Scholar]
- Yekta, S., Shih, I.H., and Bartel, D.P. (2004). MicroRNA-directed cleavage of HOXB8 mRNA. Science 304 594–596. [DOI] [PubMed] [Google Scholar]
- Yoo, B.C., Kragler, F., Varkonyi-Gasic, E., Haywood, V., Archer-Evans, S., Lee, Y.M., Lough, T.J., and Lucas, W.J. (2004). A systemic small RNA signaling system in plants. Plant Cell 16 1979–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker, M. (2003). Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.