Abstract
Human mitochondrial respiration is distinct from that of most plants, microorganisms and even some metazoans in that it reduces molecular oxygen only through the highly cyanide-sensitive enzyme cytochrome c oxidase. Here we show that expression of the cyanide-insensitive alternative oxidase (AOX), recently identified in the ascidian Ciona intestinalis, is well tolerated by cultured human cells and confers spectacular cyanide resistance to mitochondrial substrate oxidation. The expressed AOX seems to be confined to mitochondria. AOX involvement in electron flow is triggered by a highly reduced redox status of the respiratory chain (RC) and enhanced by pyruvate; otherwise, the enzyme remains essentially inactive. AOX expression promises to be a valuable tool to limit the deleterious consequences of RC deficiency in human cells and whole animals.
Keywords: cyanide resistance, alternative oxidase, human cells, Ciona intestinalis
Introduction
Since the pioneering work of Otto Warburg in 1919 (Warburg, 1919), it has been known that cyanide-resistant respiration differentiates most plants and microorganisms from mammals and other higher animals. Cyanogenic compounds are thus among the most frequently encountered poisons in nature to resist animal predators (Tattersall et al, 2001). Plants and microorganisms are endowed with various components conferring cyanide resistance, including an unusual, cyanide-resistant mode of respiration. This alternative respiration generally relies on the presence of a unique protein, the so-called alternative oxidase (AOX), that conveys electrons directly from the quinone pool of the mitochondrial respiratory chain (RC) to oxygen, hence by-passing entirely the cytochrome segment of the chain (Fig 1A; Affourtit et al, 2002). By doing so, it strongly diminishes proton extrusion linked to substrate oxidation, concomitantly decreasing ATP production. In at least some plants, this seems to prevent the repression of mitochondrial substrate oxidation by high ATP levels resulting from the phosphorylating activity of chloroplasts (Rustin & Queiroz-Claret, 1985). In addition, AOX is considered to act as an antioxidant protein by preventing over-reduction of the mitochondrial quinone pool, which is known to favour superoxide production (Maxwell et al, 1999; Lam et al, 2001). In plants, any significant involvement of the AOX protein in electron flow is triggered only by very specific conditions. First, it requires a pronounced reduction of the quinone pool, owing to the low affinity of the AOX for its quinol substrate (Bahr & Bonner, 1973). Second, the enzyme is regulated by the presence of a subset of organic acids, chiefly pyruvate, probably by increasing its substrate affinity (Millar et al, 1993; Umbach et al, 2002). A reduced redox status of the RC and a high pyruvate level are the exact conditions, resulting from inherited human metabolic disorders affecting the cytochrome segment of the mitochondrial RC (Munnich et al, 2001). On the basis of this observation, it has been a long-standing goal to express AOX in human cells, with the aim of achieving a potential rescue of electron flow and mitigating the deleterious consequences of pathological RC deficiency.
Figure 1.
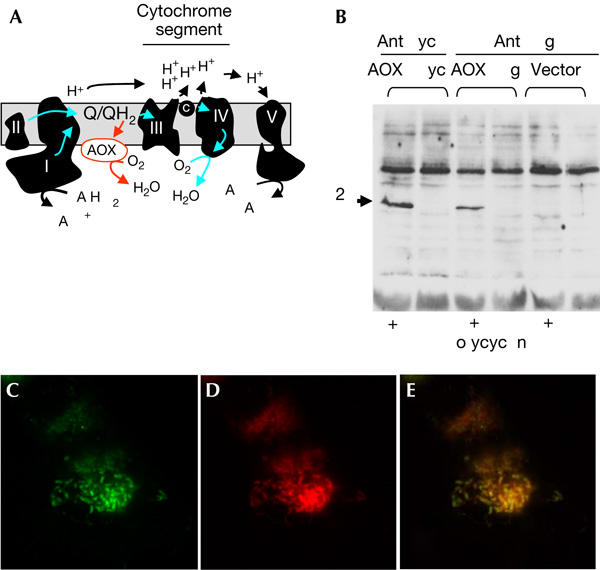
Alternative oxidase is expressible in human cells and targeted to mitochondria. (A) Simplified biochemical scheme of mitochondrial respiration and by-pass of the cytochrome segment provided by alternative oxidase (AOX). The five complexes of the respiratory chain (RC) are denoted by Roman numerals. (B) Immunoblot of 20 μg total cell lysate from Flp-In™ T-REx™-293 (Invitrogen) cell clones transfected either with AOX–Flag or AOX–Myc constructs, or empty vector, and probed with primary antibodies is shown. Lanes denoted (+) were lysates from cells treated with 1 μg/ml doxycyclin to induce transgene expression. Primary antibodies used were mouse anti-Myc monoclonal 9E10 (anti-Myc) and anti-Flag M2 antibody (anti-Flag). (C–E) Fluorescence micrographs of cells transfected with the AOX–Flag construct. (C) Immunocytochemistry using anti-Flag M2 primary antibody. (D) Staining with Mitotracker® Red (Molecular Probes). (E) Superposition of the images from (C,D). Immunocytochemistry was carried out as described by Garrido et al (2003).
Results and Discussion
Recently, a genome database search by Vanlerberghe and colleagues (McDonald & Vanlerberghe, 2004) has unexpectedly shown the occurrence of AOX in several animal phyla. This offered a potential route to expression of AOX in human cells, as the enzyme is much more likely to be well adapted to the metabolic conditions pertaining inside mammalian mitochondria than the plant enzyme (previous attempts to express plant AOX genes in human cells led to apparently uncontrolled lethality; P. Rustin, unpublished data). AOX complementary DNA (cDNA) from the ascidian Ciona intestinalis was therefore ligated directly into the doxycyclin-inducible mammalian vector pCDNA5/FRT/TO, either with or without an epitope tag. Human embryonic kidney (HEK)293T-derived T-REx cells were then transfected with the expression constructs or with the empty vector. Cells surviving treatment with antibiotics (150 μg/ml hygromycin and 15 μg/ml blasticidin), containing a single copy of the AOX transgene (or empty vector) inserted in a precise chromosomal location (see www.invitrogen.com for full explanation of the Flp-In™ T-REx expression system), were induced to express AOX by adding 1 μg/ml doxycyclin to the medium.
After 24 h of induction, AOX expression was confirmed by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and immunoblotting (Fig 1B). Both of the epitope-tagged versions of C. intestinalis AOX were detected, migrating at the size predicted by the cDNA sequence (42 kDa) after mitochondrial import. As a prerequisite for function, the AOX protein has to be targeted to mitochondria. This was verified by immunocytochemistry, in which the signal generated by flag-tagged AOX overlapped that of Mitotracker® Red, a mitochondrial marker (Fig 1C–E). We observed a similar overlap with the Myc-tagged version of the protein (not shown).
We next compared the respiratory properties at 37°C of cells harbouring either the tagged or untagged version of AOX or the empty vector, after 48 h of induction. Similar to the nontransfected parental cell line, respiration of cells harbouring the empty vector was fully sensitive to 100 μM potassium cyanide (Fig 2A, trace a). In contrast, the respiration of cells induced to express AOX, whether tagged or untagged, consistently showed from 60% to 80% resistance to cyanide (Fig 2A, trace b). A threefold increased concentration of potassium cyanide did not result in any further inhibition. The cyanide-resistant respiration was fully inhibited by the subsequent addition of 100 μM propyl gallate, a specific inhibitor of the AOX in plant mitochondria (Siedow & Bickett, 1981). We next studied the oxidation of a mitochondrial substrate, succinate, in digitonin-permeabilized cells. The oxidation of succinate by control cells (in the presence of rotenone to avoid production of any inhibitory oxaloacetate) was fully sensitive to cyanide (Fig 2A, trace c). In contrast, succinate oxidation by cells induced to express AOX was significantly resistant to cyanide, up to 60% (Fig 2A, trace d). It is important to note that propyl gallate addition in the absence of cyanide caused at most a 5–10% inhibition of oxygen uptake (Fig 2A, trace f), suggesting that AOX was only slightly active under such conditions. The residual succinate oxidation was fully inhibited by 100 μM cyanide. A detailed analysis of RC complex activities, carried out on control and AOX-expressing cells in the absence of cyanide, detected no significant effects on the activity of any of the complexes as a result of AOX expression (Table 1).
Figure 2.
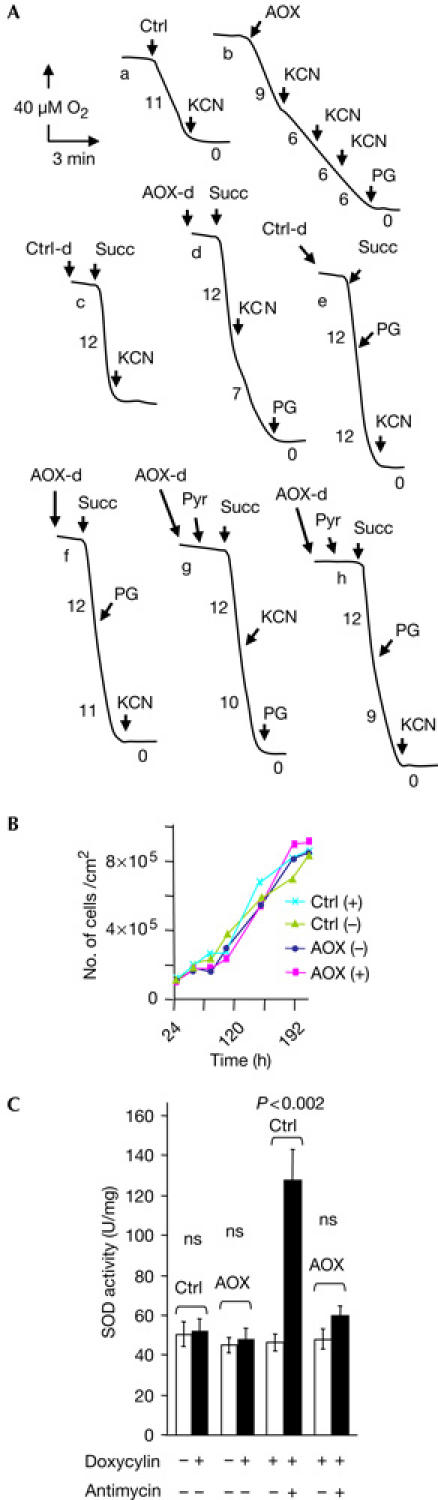
Alternative oxidase (AOX) expression modifies mitochondrial biochemistry in human cells. (A) Oxygen electrode traces after 48 h doxycyclin induction for whole cells (traces a,b) and for digitonin-permeabilized (Ctrl-d, AOX-d) cells (traces c–h) on addition of various organic acids and inhibitors as described in the text. AOX, cells transfected with untagged alternative oxidase construct; Ctrl, cells transfected with the empty vector. Cell respiration and succinate oxidation were measured using a Clark oxygen electrode. KCN, 100 μM potassium cyanide; PG, 10 μM n-propyl gallate; Pyr, 10 mM pyruvate; Succ, 10 mM succinate. Numbers along the traces represent mean values from three experiments (nmol/min per mg protein). (B) Cell growth curves: AOX, cells transfected with untagged AOX construct; Ctrl, cells transfected with empty vector, grown in standard DMEM medium supplemented with uridine and pyruvate plus (+) or minus (−) doxycyclin. (C) Superoxide dismutase (SOD) activity of empty vector-transfected and untagged AOX-transfected cells grown either with (+) or without (−) doxycyclin induction, minus (−) or plus (+) 60 μM antimycin. ns, not significant.
Table 1.
RC complex activities in control and untagged AOX-expressing cells
|
Activity (nmol/min per mg protein) |
||
|---|---|---|
| Control cells | Untagged AOX cells | |
| NADH:ubiquinone oxidoreductase (cI) |
10±1.2 |
9±1.0 |
| Succinate:cytochrome c oxidoreductase (cII+cIII) |
57±8 |
51±8 |
| Glycerol 3 phosphate:cytochrome c oxidoreductase (G3PDH+cIII) |
14±3 |
17±3.5 |
| Ubiquinol:cytochrome c oxidoreductase (cIII) |
338±46 |
297±55 |
| Cytochrome c oxidase (cIV) |
181±22 |
183±24 |
| Oligomycin-sensitive ATPase (cV) | 88±12 | 90±12 |
| AOX=alternative oxidase; NADH=nicotinamide adenine dinucleotide (reduced form). | ||
| Activities were measured under standard conditions as described previously (Rustin et al, 1994). Values are means±1 s.d. (n=3). | ||
On the basis of the differential effect of cyanide on cell respiration in vitro, we tested culture media for pH change, a widely recognized marker for lactic acid accumulation (Fig 3), in the presence or absence of cyanide during cell growth. The medium was acidified by approximately 1 pH unit by control cells grown in the presence of 1 mM cyanide for 24 h, yielding a bright lemon-yellow colour, whereas under similar conditions, the medium of the AOX-expressing cells decreased by only 0.25–0.3 pH units. This indicated that one important hallmark feature of an RC deficiency can actually be ameliorated by the presence of the AOX. Surprisingly, although the AOX supposedly works at much lower temperature in C. intestinalis, a cold seawater organism, the protein expressed in human cells is readily active and stable at 37°C.
Figure 3.
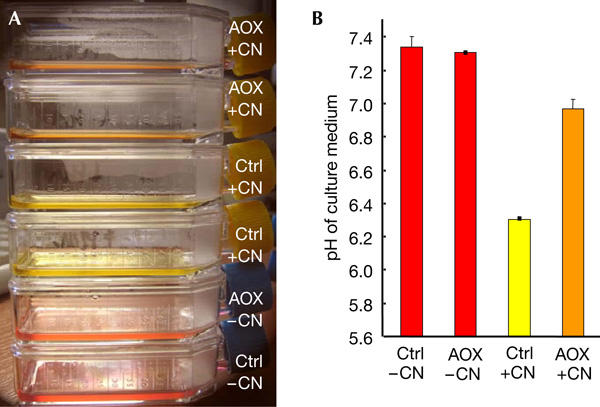
pH change in culture media of control and untagged alternative oxidase cells, treated or untreated with 1 mM potassium cyanide (KCN). Similar cell numbers (control or untagged alternative oxidase (AOX), 35,000/cm2) were seeded and grown for 24 h under standard conditions in the presence of doxycyclin. After 24 h, KCN (1 mM) was added to air-tight 25 cm2 flasks resulting (A) in a spectacular colour change to lemon-yellow in control cells treated with cyanide, as compared with a red-orange shift in AOX cells. (B) Measurement of pH values in the different supernatants confirmed the substantial acidification occurring in control cells as compared with AOX-expressing cells in the presence of cyanide. Cell numbers were similar after 24 h treatment with KCN, although morphological changes were more pronounced in control cells.
A constitutively active, nonphosphorylating AOX could be detrimental to cell survival, as it could significantly decrease the ATP produced by mitochondria. We therefore tested the effect of expressing AOX on cell growth (Fig 2B) and acidification of the medium. We did not observe any difference between the growth of cells expressing AOX and control cells (up to four cell passages, 18 days). Decreasing glucose to 0.5 mM or totally depleting it in the culture medium severely but similarly affected the growth of both control and AOX-expressing cells, HEK cells being known to be highly dependent on glucose (Siegwart et al, 1999). This suggested that under these conditions, glycolytic ATP is crucial to cell growth. In addition, there was no change in medium acidification provoked by lactate excretion in the absence of cyanide (Fig 3; other data, not shown), indicating that there was no detectable shift in the relative use of glycolysis versus mitochondrial respiration in AOX-expressing cells. This is consistent with the interpretation that, under normal conditions, electron flow uses the phosphorylating cytochrome segment and AOX is essentially inactive, and hence the rate of the succinate-cytochrome c reductase activity measured in vitro (Table 1) is unchanged. This activity would be expected to show a significant decrease if electrons were readily conveyed directly to oxygen by an active AOX.
In addition, AOX has been shown in other organisms to act as an antioxidant enzyme by preventing the superoxide production resulting from a highly reduced quinone pool (Maxwell et al, 1999). Persistently active AOX should thus result in decreased superoxide production and lead to a decreased level of the inducible superoxide dismutase (SOD) activity (Geromel et al, 2001). We therefore compared SOD activity in the induced and noninduced AOX cells and found no significant difference (Fig 2C). Taken together, these data, replicated on both the epitope-tagged (Myc or Flag) and untagged AOX versions, support the view that the enzyme remains inactive as long as the mitochondrial quinone pool is not highly reduced, that is, as long as the cytochrome segment of the RC remains functional.
We next investigated the effect of AOX expression on antimycin-induced superoxide overproduction by the RC, as detected by the induction of SOD (Geromel et al, 2001). As predicted, a 16 h treatment with 60 μM antimycin induced a roughly threefold increase in SOD activity in control cells (Fig 2C). In contrast, no significant increase could be observed in AOX-expressing cells, indicating that superoxide overproduction resulting from antimycin inhibition of complex III is alleviated by AOX expression. Although no significant changes in SOD induction were observed in cells grown in the presence of oligomycin, AOX expression conferred significant protection against oligomycin-induced cell death: after a 6 h treatment with 30 μM oligomycin, only 20% of control cells (23±8%; n=3) were still adherent, as compared with 60% of AOX-expressing cells (61±16%; n=3).
As pyruvate is known to act as an allosteric regulator of plant mitochondrial AOX, we finally attempted to determine whether this organic acid, of great importance in mitochondrial diseases (Stacpoole et al, 1978), also affected C. intestinalis AOX expressed in human cells. We therefore compared the cyanide sensitivity of succinate oxidation under state 4 conditions in permeabilized AOX-expressing cells in the absence or presence of pyruvate, plus rotenone. This latter inhibitor, specific to complex I, was added to block the nicotinamide adenine dinucleotide (reduced form) (NADH) re-oxidation required for sustained oxidation of the added pyruvate. In the presence of pyruvate, we observed a consistent increase in cyanide-resistant succinate oxidation, from approximately 60% to 80% (Fig 2A, compare traces g and d). This strongly suggests that the expressed C. intestinalis AOX was subjected to a similar allosteric regulation by organic acid as the plant enzyme, despite the absence of the supposedly critical cysteine residue in the predicted amino-acid sequence (McDonald & Vanlerberghe, 2004). In agreement with this inference, we observed that n-propyl gallate, added in the absence of cyanide but in the presence of pyruvate, brings about a more pronounced (approximately 20%) inhibition of succinate oxidation (Fig 2A, compare traces h and f). This suggested that both the level of quinone reduction and activation by pyruvate control the involvement of the AOX in mitochondrial substrate oxidation.
The successful expression of C. intestinalis AOX in human cells constitutes a promising tool to study further the consequences of RC dysfunction, because it offers a unique possibility to disconnect electron flow through most of the RC from the phosphorylation process. In the longer term, allotopic expression of AOX may provide an effective therapy for, at present, intractable RC diseases. The first step in this endeavour should be the expression of AOX in whole organism models, for example, mouse or Drosophila, exhibiting RC deficiency.
Methods
Construction of AOX-expressing vector. For the construction of epitope-tagged expression vectors, annealed oligonucleotide pairs GJ247: 5′-GGCCGCGGAACAAAAACTCATCTCAGAAGAGGATC TGTGATGA-3′ plus GJ248: 5′-TCGATCATCACAGATCCTCTTCTGAGATGAGTTTT TGTTCCGC-3′ (Myc), and GJ249: 5′-GGCCGCGGATTACAAGGATGACGACGATAAGTGA-3′ plus GJ250: 5′-TCGATCACTTATCGTCGTCATCCTTGTAATCCGC-3′ (Flag) were ligated into pCDNA5/FRT/TO (Invitrogen, Carlsbad, CA, USA) digested with NotI and XhoI. pBluescriptII clones carrying overlapping stretches of the C. intestinalis AOX cDNA (cieg032g14 and cic1022c03, http://ghost.zool.kyoto-u.ac.jp/indexr1.html) were used to assemble a full-length cDNA by PCR, using primer pairs GJ241: 5′-GGGAAGCTTCCACCATGTTGTCTACCGGAAGTAAA AC-3′ plus GJ242: 5′-GGGGTACCGAGAGTATAACCAGAAAAAAC-3′ on cieg032g14, and GJ243: 5′-GGTACCTACACTGGACGGCTAGATGAG-3′ plus GJ244: 5′-GGGGCGGCCGCTTGTCCAGGTGGATAAGGATTC-3′ or GJ 245: 5′-GGGGCGGCCGCTATTGTCCAGGTGGATAAGGATTC-3′ on cic1022c03. After sequence verification, the subcloned amino- and carboxy-terminal fragments were ligated into pCDNA5/FRT/TO, or the modified, epitope tag-containing vectors, as HindIII–KpnI and KpnI–NotI fragments, respectively.
Cell culture and transfection Flp-In™ T-REx™-293 cells (Invitrogen) were cultured in standard DMEM medium supplemented with 200 μM uridine, 1 mM pyruvate and 5% TET-free fetal bovine serum (Ozyme, St Quentin en Yvelines, France) plus appropriate antibiotics for transgene selection, and were transfected using Lipofectamine™ (Invitrogen) according to the manufacturer's instructions. AOX expression was induced by treating cells with 1 μg/ml doxycyclin.
Fluorescence microscopy Immunocytochemistry was carried out as described (Garrido et al, 2003). AOX-expressing cells were analysed using anti-Flag M2 primary antibody (Stratagene, La Jolla, CA, USA) combined with Mitotracker® Red (Molecular Probes, Eugene, OR, USA) staining.
Biochemical methods. Cell lysates were prepared and analysed for AOX expression by immunoblotting after SDS–PAGE. Primary antibodies used were mouse anti-Myc monoclonal 9E10 (Roche Molecular Biochemicals, Nutley, NJ, USA) and anti-Flag M2 antibody (Stratagene). Peroxidase-conjugated goat anti-mouse IgG (Vector Laboratories Inc.) was used as a secondary antibody (Spelbrink et al, 2000). Cell respiration and succinate oxidation in digitonin-permeabilized cells were measured after 48 h doxycyclin induction, using a Clark oxygen electrode (Hansatech, UK) fitted to a magnetically stirred 250 μl chamber maintained at 37°C in 250 μl of a medium consisting of 0.3 M mannitol, 5 mM KCl, 5 mM MgCl2, 10 mM phosphate buffer (pH 7.2) and 1 mg/ml bovine serum albumin, plus substrates or inhibitors as shown in the legend to Fig 2. Total SOD activity (EC 1.15.1.1; Mn- and CuZn-dependent enzymes) was determined by the pyrogallol autoxidation assay, 50% decrease of the autoxidation rate by SOD being defined as 1 U (Roth & Gilbert, 1984). Results were expressed as U/mg protein.
Acknowledgments
We thank Y. Kohara for kindly providing C. intestinalis AOX cDNA subclones, S. Wanrooij and M. Jokela for assistance with transfection and cell culture and P. Martinsson for guidance with cell imaging. This work was supported by Institute of National de la Santé et de la Recherche Médicale, Association Française contre les Myopathies (Project No. 11639), the Academy of Finland, Tampere University Hospital Medical Research Fund, Juselius Foundation and the European Union (EUMITOCOMBAT project).
References
- Affourtit C, Albury MS, Crichton PG, Moore AL (2002) Exploring the molecular nature of alternative oxidase regulation and catalysis. FEBS Lett 510: 121–126 [DOI] [PubMed] [Google Scholar]
- Bahr JT, Bonner WD Jr (1973) Cyanide-insensitive respiration. II. Control of the alternate pathway. J Biol Chem 248: 3446–3450 [PubMed] [Google Scholar]
- Garrido N, Griparic L, Jokitalo E, Wartiovaara J, van der Bliek AM, Spelbrink JN (2003) Composition and dynamics of human mitochondrial nucleoids. Mol Biol Cell 14: 1583–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geromel V, Kadhom N, Cebalos-Picot I, Ouari O, Polidori A, Munnich A, Rotig A, Rustin P (2001) Superoxide-induced massive apoptosis in cultured skin fibroblasts harboring the neurogenic ataxia retinitis pigmentosa (NARP) mutation in the ATPase-6 gene of the mitochondrial DNA. Hum Mol Genet 10: 1221–1228 [DOI] [PubMed] [Google Scholar]
- Lam E, Kato N, Lawton M (2001) Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411: 848–853 [DOI] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96: 8271–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A, Vanlerberghe G (2004) Branched mitochondrial electron transport in the Animalia: presence of alternative oxidase in several animal phyla. IUBMB Life 56: 333–341 [DOI] [PubMed] [Google Scholar]
- Millar AH, Wiskich JT, Whelan J, Day DA (1993) Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Lett 329: 259–262 [DOI] [PubMed] [Google Scholar]
- Munnich A, Rötig A, Cormier V, Rustin P (2001) Clinical presentation of respiratory chain deficiency. In The Metabolic and Molecular Bases of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D (eds) 8th edn, pp 2261–2274. New York, USA: McGraw-Hill Medical Publishing Division [Google Scholar]
- Roth EF Jr, Gilbert HS (1984) The pyrogallol assay for superoxide dismutase: absence of a glutathione artifact. Anal Biochem 137: 50–53 [DOI] [PubMed] [Google Scholar]
- Rustin P, Queiroz-Claret C (1985) Changes in oxidative properties of Kalanchoe blossfeldiana leaf mitochondria during development of Crassulacean acid metabolism. Planta 164: 415–422 [DOI] [PubMed] [Google Scholar]
- Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray JM, Munnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228: 35–51 [DOI] [PubMed] [Google Scholar]
- Siedow JN, Bickett DM (1981) Structural features required for inhibition of cyanide-insensitive electron transfer by propyl gallate. Arch Biochem Biophys 207: 32–39 [DOI] [PubMed] [Google Scholar]
- Siegwart P, Cote J, Male K, Luong JH, Perrier M, Kamen A (1999) Adaptive control at low glucose concentration of HEK-293 cell serum-free cultures. Biotechnol Prog 15: 608–616 [DOI] [PubMed] [Google Scholar]
- Spelbrink JN et al. (2000) In vivo functional analysis of the human mitochondrial DNA polymerase POLG expressed in cultured human cells. J Biol Chem 275: 24818–24828 [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Moore GW, Kornhauser DM (1978) Metabolic effects of dichloroacetate in patients with diabetes mellitus and hyperlipoproteinemia. N Engl J Med 298: 526–530 [DOI] [PubMed] [Google Scholar]
- Tattersall DB, Bak S, Jones PR, Olsen CE, Nielsen JK, Hansen ML, Hoj PB, Moller BL (2001) Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 293: 1826–1828 [DOI] [PubMed] [Google Scholar]
- Umbach AL, Gonzalez-Meler MA, Sweet CR, Siedow JN (2002) Activation of the plant mitochondrial alternative oxidase: insights from site-directed mutagenesis. Biochim Biophys Acta 1554: 118–128 [DOI] [PubMed] [Google Scholar]
- Warburg O (1919) Über die Geschwindigkeit der photochemischen Kohlen-saürezersetzung in lebenden Zellen. Biochem Z 100: 230–270 [Google Scholar]


