Abstract
Cell interactions mediated by Notch family receptors have been implicated in the specification of tissue boundaries. Tightly localized activation of Notch is crucial for the formation of sharp boundaries. In the Drosophila wing imaginal disc, the Notch receptor is expressed in all cells. However, Notch activity is limited to a narrow stripe of cells along the dorsal–ventral compartment boundary, where it induces the expression of target genes. How a widely expressed protein becomes tightly regulated at the dorsal–ventral boundary in the Drosophila wing is not completely understood. Here, we show that the transmembrane protein Crumbs is involved in a feedback mechanism used by Notch to refine its own activation domain at the Drosophila wing margin. Crumbs reduces the activity of the γ-Secretase complex, which mediates the proteolytic intracellular processing of Notch. These results indicate a novel molecular mechanism of the regulation of Notch signal, and also that defects in Crumbs might be involved in similar abnormal γ-Secretase complex activity observed in Alzheimer's disease.
Keywords: Drosophila wing, Crumbs, Notch, presenilin
Introduction
Notch signalling is involved in a variety of cell-fate decisions during development. Ligand binding to the Notch receptor results in a γ-Secretase-mediated proteolytic intracellular processing of Notch. The Notch intracellular domain fragment (Nicd) translocates to the nucleus, where it interacts with the Suppressor of Hairless (Su(H)) DNA-binding protein (reviewed by Lai, 2004). In cooperation with other transcriptional activators, including Mastermind, transcription of Notch target genes can be induced.
In the wing imaginal disc, Notch is activated at the boundary between dorsal and ventral compartments. Several mechanisms have been implicated in limiting Notch activity to cells immediately adjacent to the dorsal–ventral (DV) boundary. Early in development, restricted expression of the glycosyltransferase Fringe in dorsal cells modifies the receptor protein Notch in the dorsal compartment (Bruckner et al, 2000; Moloney, 2000). Fringe activity makes dorsal cells more sensitive to Delta, a ligand expressed by ventral cells, and less sensitive to Serrate, the ligand expressed by dorsal cells (Fig 1A; Diaz-Benjumea & Cohen, 1995; de Celis et al, 1996; Doherty et al, 1996; Fleming et al, 1997; Panin et al, 1997). Consequently, signalling by each ligand is limited to nearby cells on the opposite side of the boundary, with the result that high levels of Notch activity are limited to a narrow band of cells along the DV boundary. Later in development, another set of cell interactions takes over to maintain Notch activity along the DV boundary (Fig 1A; de Celis & Bray, 1997; Micchelli et al, 1997). Notch activation induces Wingless expression in boundary cells. Wingless expression limits Notch activity to cells immediately adjacent to the DV boundary.
Figure 1.
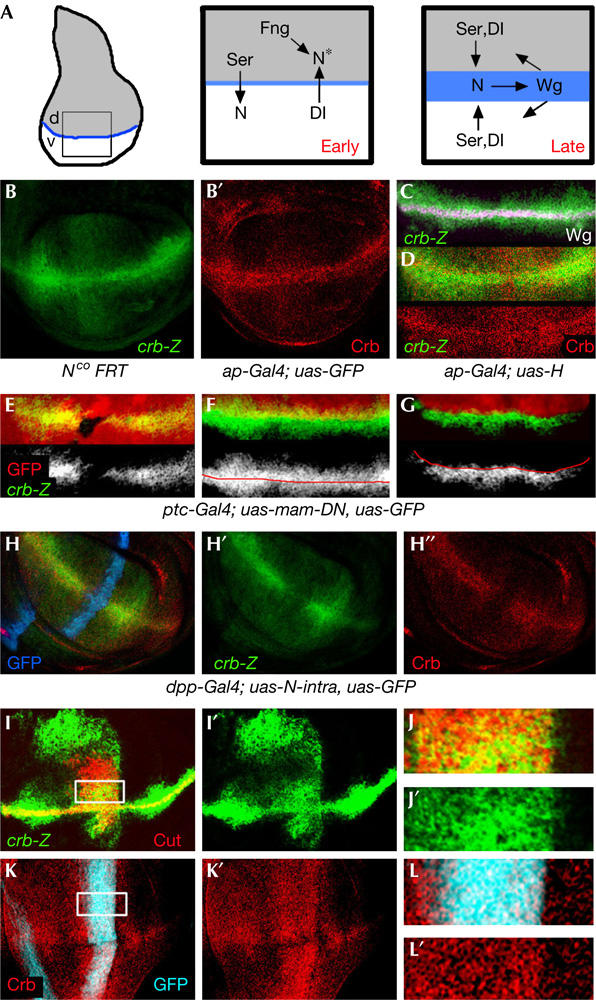
crb expression in the wing imaginal disc depends on Notch activity. (A) Establishment and maintenance of the dorsal–ventral (DV) organizing centre. Early in development, Serrate (Ser) signals to ventral cells and Delta to dorsal cells to activate Notch along the DV boundary. Fng modifies Notch (N*) in dorsal cells, thus making it sensitive to Dl and not to Serrate. Later in development, a positive feedback loop between Wg- and Ser/Dl-expressing cells maintains the signalling centre along the DV boundary. (B–D) Crumbs protein (red) and crb-lacZ expression (green) in third instar wing discs. crb-lacZ expression was visualized by an antibody against β-galactosidase (β-Gal). Wg protein expression is shown in white in (C). (E) Clones of cells lacking Notch activity marked by the absence of green fluorescent protein (GFP; red). crb-lacZ expression (green) is reduced in the mutant clones. (F,G) Wing discs that expressed GFP (F, in red) or Hairless and GFP (G) under the control of apGal4. crb-lacZ expression (green) is reduced in the apGal4 expression domain. (H–H″) Wing disc that expressed GFP (blue) and a dominant-negative form of Mastermind (mam-DN) under the control of ptcGal4. crb-lacZ expression (green) and Crb protein (red) are reduced in the ptcGal4 expression domain. (I,I′) Wing disc that expressed the intracellular domain of Notch under the control of dppGal4. crb-lacZ expression is shown in green and Cut protein expression in red. (J,J′) High magnification of the squared region shown in (I). (K,K′) Wing disc that expressed the intracellular domain of Notch and GFP (blue) under the control of dppGal4. Crumbs protein expression is shown in red. (L,L′) High magnification of the squared region of the disc shown in (K).
The transmembrane protein Crumbs is a central regulator of epithelial apical–basal polarity in Drosophila, and loss-of-function mutations in the human homologue of Crumbs, CRB1 (RP12), cause recessive retinal dystrophies, including retinitis pigmentosa (den Hollander, 1999). Here, we present evidence of a new function of Crumbs in Drosophila. Crumbs mediates a novel negative feedback loop of Notch to restrict its own activation domain to a thin stripe corresponding to the DV boundary. We show that Crumbs attenuates Notch signalling by repressing the activity of the γ-Secretase complex. This complex also mediates the intracellular cleavage of the amyloid precursor protein (APP), thus leading to accumulation of the Aβ peptide in plaques in Alzheimer's disease (AD). We postulate that Crumbs may also be a potential modulator of AD pathogenesis.
Results and Discussion
We were interested in the function of Crumbs (Crb) on the basis of its expression pattern in the developing wing imaginal disc. A piggyBac-lacZ insertion (see Methods for details) that lies 240 bp upstream of the crumbs transcription start site is expressed at higher levels along the DV boundary (Fig 1B–D). Low level of expression is detected in all wing cells. Expression of Crb protein coincides with that of crb-lacZ expression (Fig 1B,D), which depends on Notch activity. Clones of cells mutant for the Notch receptor reduce crb expression (Fig 1E). Blocking Notch activation by driving the expression of Hairless (a repressor of the Notch signalling pathway at a transcriptional level) or a dominant-negative form of the nuclear Notch effector Mastermind (Giraldez et al, 2002) reduces expression of crb in a cell-autonomous manner (Fig 1F–H). Expression of an activated form of the Notch receptor (Nicd) in a perpendicular stripe to the DV boundary induces ectopic crb expression in the wing pouch in a cell-autonomous manner (Fig 1I–L). In wing discs mutant for the Notch gain-of-function allele AbruptexM1, the expression domain of crb is expanded and ectopically expressed in the wing pouch (Fig 3K,M).
Figure 3.

Epistatic analysis of Crb activity. (A–C) Wing discs with clones of cells lacking crb activity and marked by the absence of the green fluorescent protein (GFP; green) marker. Wg protein distribution (A–C, red) and wg-lacZ (C, blue) expression were monitored. Note ectopic expression of Wg and wg-lacZ in the cells of the clones close to the dorsal–ventral (DV) boundary (red arrows). (D–F) Wing discs with crb11A22 mutant clones positively labelled by the GFP (green) marker. Activity of the Notch signalling pathway was blocked in these clones using a dominant-negative form of Mastermind (Mam-DN; D), Serrate ligand (Ser-DN; E) and Notch receptor (Necd; F). Note the absence of ectopic expression of Wg in the clones (red arrowheads). (G,H) Wing discs with crb11A22 mutant clones positively labelled by the GFP marker and expressing the full-length form of Crumbs (G) or a truncated form of Crumbs lacking the intracellular domain (Crb-extra-TM; H). (I) Overexpression of Crb-extra-TM (green) in the ptc-Gal4 domain leads to reduced expression of Cut (red) at the DV boundary (red arrow). (J–M) Wingless (J,K) and Cut (L,M) protein expression (red) in AxM1 (J,L) and AxM1; crumbsM11.M2-lacZ/+ third instar wing imaginal discs (K,M). crumbsM11.M2-lacZ expression is shown in green.
We noticed that the expression domain of crb is broader than that of Wingless (Wg) or Cut, two known target genes of Notch at the DV boundary (Fig 1C; data not shown). This observation suggests that cut and wg may be high-threshold targets of Notch, and crumbs may require lower levels of Notch activation to be induced. Indeed, crb responds better to ectopic activation of the Notch signalling pathway than Wg and Cut. In AbruptexM1 (Fig 3J–M) and dpp-gal4; uas-Nicd wing discs (Fig 1I), the regions of ectopic expression of crb are larger than those of Cut and Wg. We monitored Cut, Wg and Crb expression in a thermosensitive Notch background (Nts2) reared for 24 h at permissive (18°C) or restrictive (25 and 29°C) temperatures. At 18°C, all target genes are expressed along the DV boundary (supplementary Fig 1 online). At 25°C, Cut expression is almost completely lost, and Wg and Crb expression levels are slightly reduced. At 29°C, expression of Cut and Wg is not observed along the DV boundary, and expression levels of Crb are reduced. When reared at 29°C for longer periods, Crb expression is lost.
The crb gene was genetically identified as an essential component for organizing apical–basal polarity and adherens junctions (AJs) in embryonic epithelia (Tepass et al, 1990). Crb is a transmembrane protein with a long extracellular domain with 28 EGF repeats and a short cytoplasmic tail. This intracellular domain is required and is sufficient to exert Crb function in setting apical–basal polarity. To assess the role of Crb in wing development, clones of crb mutant cells were generated and analysed in wing discs and adult wings. Mutant clones for crb (crb1 and crb11A22) are able to cover large areas of the wing, indicating that loss of crb does not compromise cell viability. Apical–basal polarity and AJs are not affected in these clones, as previously reported in the eye imaginal disc (Izaddoost et al, 2002; Pellikka et al, 2002). Localization of Armadillo and E-Cadherin proteins, two components of the AJs, is not affected (supplementary Fig 2 online). Thus, the maintenance of apical–basal polarity and AJs in the wing epithelia does not seem to depend on Crb activity. When abutting the DV boundary, crb mutant clones produce a broadening of the wing margin in a cell-autonomous manner (Fig 2A–C), mimicking a gain-of-function activity of Notch. The adult wing is decorated by five longitudinal veins (Fig 2G). Increased levels of Notch activity induce loss of vein tissue (Fig 2P). crb mutant clones lose vein differentiation in a cell-autonomous manner (Fig 2H–J). The haploinsufficient phenotype of Notch (loss of wing margin and vein thickening; Fig 2K) is rescued in a heterozygous crb mutant background (Fig 2L–N). These results indicate that Crb attenuates Notch signalling in the developing wing.
Figure 2.

crb mutants cause Notch gain-of-function phenotypes. (A) Cuticle preparation of a wild-type anterior wing margin (AWM) in the dorsal compartment. Note the single, well-organized row of mechanosensory bristles and the thin chemosensory bristles in every fourth position. (B,C) Two examples of AWM in the dorsal compartment carrying clones mutant for crb11A22 and marked with forked. Note the extra mechanosensory and chemosensory bristles. The contour of the clones is highlighted in red. (D–F) Wing discs with clones of cells lacking crb activity and marked by the absence of the green fluorescent protein (GFP; green) marker. Expression of Senseless protein (red) visualizes the domain in which the sensory organs of the wing margin will develop. Note the ectopic expression of Senseless in mutant cells (arrows). (G–J) Cuticle preparations of a wild-type wing (G) and wings with crb mutant clones running along the veins (H–J). Note the absence of vein differentiation (arrows in (H), and clones highlighted in red in (I,J)). (K–M) Cuticle preparations of Df(1)Notch-8/+ (K), Df(1)Notch-8/+; crumbs11A22/+ wing (L) and Df(1)Notch-8/+; crumbsM11M2/+ wings (M). (N) Histogram showing the number of nicks per wing in the genotypes corresponding to (K–M). Nicks per wing(Df(1)Notch-8/+)=3.3±1; nicks per wing(Df(1)Notch-8/+; crumbs11A22/+)=0.7±0.5; nicks per wing(Df(1)Notch-8/+; crumbsM11M2/+)=0.2±0.6. n(Df(1)Notch-8/+)=10, n(Df(1)Notch-8/+; crumbs11A22/+)=18, n(Df(1)Notch-8/+; crumbsM11M2/+)=11. (O) Cuticle preparation of a wing expressing a truncated form of Crumbs lacking the intracellular domain (Crb-extra-TM) in the MS1096-Gal4 domain. Note that veins are thicker and deltas are in the distal part of the veins (arrows). (P) Cuticle preparation of an AxM1/+ wing. Note the loss of the distal part of the veins (arrows).
The wing margin is decorated by sensory bristles (Fig 2A). The domain in which the precursors of sensory bristles form can be visualized in developing wing discs by the expression of the transcription factor Senseless (Nolo et al, 2000). Clones mutant for crumbs form more rows of Senseless-expressing cells (Fig 2D–F). Wg is expressed along the DV boundary by the activity of Notch. High levels of Wg signalling in nearby cells specify the domain of Senseless expression. The broadening of the Senseless domain suggests that Wg might be ectopically expressed owing to ectopic activation of the Notch signalling pathway. We then analysed Wg protein and wg-lacZ expression in clones of crb mutant cells. wg was ectopically expressed in these cells (Fig 3A–C), although at lower levels than the endogenous wg expression domain. The largely autonomous effect of crb mutant clones on bristle specification may be due to the relatively low levels of ectopic Wg. Cut, a target of Notch that requires high levels of Notch activity (supplementary Fig 1 online), was not affected in these clones (data not shown). AbruptexM1 is a gain-of-function allele of Notch, in which the expression domain of the Notch target genes Wg and Cut along the DV boundary is broader than in wild-type discs (Fig 3J,L). In crb heterozygous wing discs, this phenotype is enhanced (Fig 3K,M). These results indicate ectopic activation of the Notch signalling pathway in the absence of Crb activity.
We wondered at which level Crb affects the Notch signalling cascade. The absence of Crb activity induces hyperactivation of the Notch signalling pathway. We then tried to rescue the wing phenotype by coexpressing dominant-negative forms of the nuclear Notch effector Mastermind (Giraldez et al, 2002), the Notch receptor (Necd; Parks et al, 2000) and the Notch ligands (SerDN or DlDN; see Methods for details). Mastermind blocks Notch signalling at the transcriptional level. The dominant-negative form of Notch blocks signal reception and its ligands titrate out Notch receptor (Sun & Artavanis-Tsakonas, 1997; Parks et al, 2000; Bardot et al, 2005), thus blocking Notch signalling at the receptor level. Interestingly, in all cases, ectopic expression of Wingless was rescued, suggesting that Crb acts upstream or at the level of the Notch receptor (Fig 3D–F). The subcellular localization of Crb partially overlaps that of Notch at the Zonula Adherens (supplementary Fig 2 online). In crb mutant clones, Notch receptor subcellular localization is not affected.
Ligand binding to the Notch receptor results in a γ-Secretase-dependent proteolytic intracellular processing of Notch that gives rise to the Notch intracellular domain fragment (Nicd), which translocates to the nucleus, where it interacts with the Suppressor of Hairless (Su(H)) DNA-binding protein (Lai, 2004). We then monitored γ-Secretase activity in the absence of Crb activity. For this purpose, we used three different reporters of γ-Secretase activity. The first two reporters express, either specifically in the eye under the control of the eye-specific GMR promoter or ubiquitously under the control of heat-shock promoter, a truncated form of β-APP, a transmembrane protein also cleaved by γ-Secretase (Struhl & Adachi, 2000) in which the intracellular domain is substituted by the Gal4 protein (Struhl & Adachi, 2000). The γ-Secretase reporter output constructs consist of a Gal4-responsive transcriptional cassette driving either the expression of the Drosophila cell death activator GRIM (Guo et al, 2003) or green fluorescent protein (GFP; Struhl & Adachi, 2000). Cleavage of APP releases from the membrane a fragment consisting of the intracellular domain of APP and Gal4, which translocates to the nucleus and activates GRIM or GFP expression, thus leading to reduced or green fluorescent adult eyes (Fig 4B,D). Flies mutant for crumbs enhance γ-Secretase cleavage of APP in the adult eye, indicating that Crumbs attenuates γ-Secretase activity (Fig 4C–F). In the wing imaginal discs, γ-Secretase cleavage of APP is reduced in the domain of crb expression (Fig 4H,I). Flies heterozygous for crb enhance the activity of γ-Secretase (compare Fig 4G,H). Similar results were obtained when γ-Secretase cleavage of Notch was measured (Fig 4J–L). In this case, a reporter ubiquitously expressing a truncated form of the Notch receptor lacking the extracellular domain (N-ECN) was used. The intracellular domain was substituted by the Gal4 protein (Struhl & Adachi, 2000).
Figure 4.
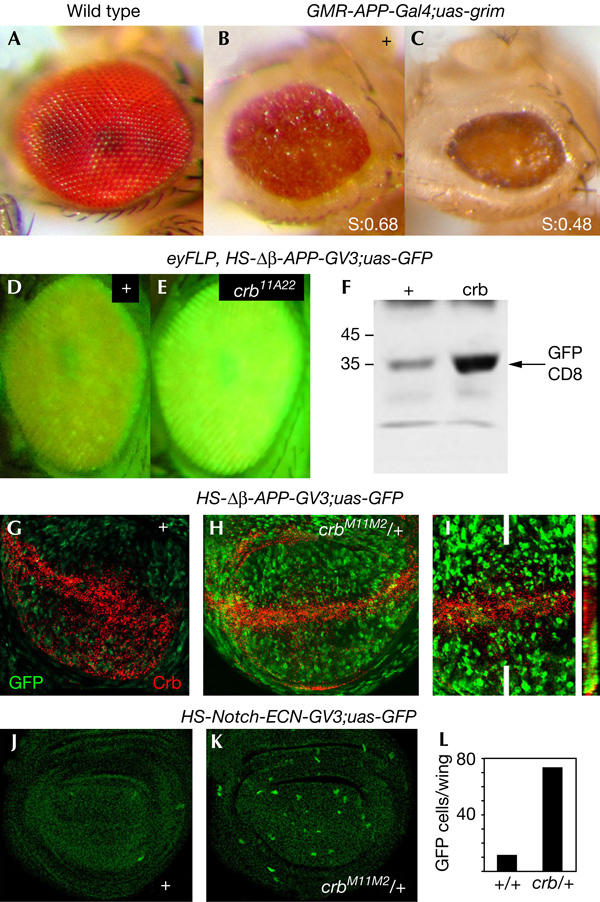
Crb reduces γ-Secretase activity. (A–C) Adult fly eyes of the following genotypes: (A) wild type; (B) GMR–APP–GAL4, UAS–GRIM/+ and (C) GMR–APP–GAL4, UAS–GRIM/crbM11.M2. The final size of the adult flies was measured and compared with wild-type eyes. The ratio (mutant eye size/wild-type eye size) is shown as S in (B,C). S(GMR–APP–GAL4, UAS–GRIM/+)=0.68±0.1, n=60; S(GMR–APP–GAL4, UAS–GRIM/crbM11.M2)=0.48±0.06, n=57. (D,E) Adult fly eyes of the following genotypes: (D) eyFLP,HS–Δβ-APP–GAL4-VP16, UAS–GFP and (E) eyFLP,HS–Δβ-APP–GAL4-VP16, UAS–GFP; FRT82/FRT82 crb11A22. (F) Western blot showing the green fluorescent protein (GFP) amounts of eyFLP,HS–Δβ-APP–GAL4-VP16, UAS–GFP (control) and eyFLP,HS–Δβ-APP–GAL4-VP16, UAS–GFP; FRT82/FRT82 crb11A22 adult heads. (G–I) HS–Δβ-APP–GAL4-VP16, UAS–GFP (G) and HS–Δβ-APP–GAL4-VP16, UAS–GFP; crbM11M2/+ wing discs (H,I). Crb protein expression (red) is shown in (G). crb-lacZ expression (red) was visualized in (H,I) by an antibody against β-galactosidase. The right panel in (I) shows an XZ confocal section of the wing disc at the level of the white lines. (J,K) HS–Notch-ECN–GAL4-VP16, UAS–GFP (J) and HS–Notch-ECN–GAL4-VP16, UAS–GFP; crbM11M2/+ wing discs (K). (L) Histogram showing the number of GFP-positive cells per wing in the genotypes corresponding to (J,K). The number of discs per genotype was 6.
Crumbs associates with the Stardust and DPATJ proteins through its short cytoplasmic tail to establish apical–basal cell polarity in the embryo (Bhat et al, 1999; Bachmann et al, 2001). Expression of this cytoplasmic tail in a mutant background for crb is sufficient to partially rescue the failure in apical–basal cell polarity, indicating that the large extracellular domain of Crb is dispensable for this process. Three different observations indicate that the extracellular domain of Crb is required to attenuate Notch signalling and that the intracellular domain is dispensable. Mutant clones for a null allele of stardust (sdtXP96) are able to cover large areas of the wing without any overt phenotype when abutting the DV boundary or when running along the longitudinal veins (data not shown). The crb mutant wing phenotype can be rescued when simultaneously expressing either full-length Crb (Fig 3G) or a truncated form of Crb lacking the whole intracellular tail (Crb-Extra-TM; Fig 3H). Overexpression of Crb-Extra-TM leads to a mild downregulation of the Notch signalling pathway (Figs 2O, 3I). In the adult wing, veins are thicker, resembling a Notch loss-of-function phenotype (de Celis & Garcia Bellido, 1994). In the wing imaginal disc, Crb-Extra-TM overexpression reduces the expression levels of Cut at the DV boundary, a target of Notch that requires high levels of Notch activity. Wg expression is not affected (data not shown).
Signalling centres along compartment boundaries are required to organize the growth and pattern of the surrounding tissue. However, too much of a signal has deleterious effects. The Notch signalling centre organizes the growth and pattern of the developing wing primordium, partially through the secreted protein Wingless. Wingless activity contributes to limit Notch activity to cells immediately adjacent to the DV boundary. Here, we present evidence that Notch also contributes to the refinement of its activation domain through its target gene crumbs. Crumbs attenuates Notch signalling by repressing the activity of the γ-Secretase complex. Many loss-of-function mutations in the human homologue of Crumbs, CRB1, cause recessive retinal dystrophies, including retinitis pigmentosa (den Hollander, 1999). Given the fact that the γ-Secretase complex also mediates the intracellular cleavage of the transmembrane protein APP, leading to accumulation of the Aβ peptide in plaques in AD, we postulate that Crumbs may also be involved in modulating AD pathogenesis. Our analysis indicates a role for the extracellular part of the Crb protein in this process. It is interesting to note that many mutations that give rise to retinal dystrophies are missense mutations that affect different EGF or LG domains of CRB1 (den Hollander, 1999). Thus, molecular interactions mediated by the extracellular domain of Crb may be crucial in both types of disease.
Methods
Drosophila strains. crbM11.M2 (crb-lacZ in the text) is a piggyBac-lacZ insertion 240 bp upstream of the crumbs transcription start site. Homozygous crbM11.M2 embryos show a strong crumbs loss-of-function phenotype (data not shown). The piggyBac-lacZ reporter plasmid was constructed as described in supplementary Methods. EP-mamDN is described by Giraldez et al (2002). UAS-SerDN and UAS-DlDN were constructed as described in supplementary Methods. Other stocks are described in FlyBase. Genotypes of larvae used for genetic mosaic analyses are described in supplementary Methods.
Antibodies. Rat anti-Crb was a gift from Choi (Izaddoost et al, 2002). Guinea-pig anti-Senseless was a gift from H. Bellen (Nolo et al, 2000). Other antibodies are commercially available.
Monitoring γ-Secretase activity in Drosophila. Three different reporter constructs were used. The first reporter construct consists of the full-length APP and the yeast transcription factor GAL4 appended to the APP carboxyl terminus. It is expressed in the adult eye under the control of the GMR promoter (Guo et al, 2003). The second and third reporter constructs consist of truncated forms of APP (Δβ-APP) or Notch (Notch-ECN) lacking the extracellular domain and the transmembrane domain joined to a Gal4–VP16 fusion protein (Struhl & Adachi, 1998). They are expressed under the control of the hsp70 promoter. Induction of expression is carried out as described by Struhl & Adachi (2000). The γ-Secretase reporter output constructs consist of Gal4-responsive transcriptional cassettes driving the expression of the Drosophila cell death activator GRIM or the GFP protein.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400617-s1.jpg, http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400617-s2.jpg, http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400617-s3.pdf).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 1
Supplementary Material
Acknowledgments
We thank M. Averof, in whose lab the M11.M2 line was identified. We thank H. Bellen, S. Campuzano, K.-W. Choi, S. Cohen, M. Guo, E. Knust, G. Struhl, U. Tepass and the Bloomington Stock Center for reagents and J. Casanova, S. Cohen, C. Dotti and three anonymous reviewers for comments on the manuscript. H.H. is a recipient of a Juan de la Cierva postdoctoral contract. Work in the laboratory of M.M. is funded by a grant from the Dirección General de Investigación Científica y Técnica (BFU2004-00167/BMC) and a European Union research contract LSHM-CP-2003-503330 (APOPIS).
References
- Bachmann A, Schneider M, Theilenberg E, Grawe F, Knust E (2001) Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature 414: 638–643 [DOI] [PubMed] [Google Scholar]
- Bardot B, Mok LP, Thayer T, Ahimou F, Wesley C (2005) The Notch amino terminus regulates protein levels and Delta-induced clustering of Drosophila Notch receptors. Exp Cell Res 304: 202–223 [DOI] [PubMed] [Google Scholar]
- Bhat MA, Izaddoost S, Lu Y, Cho KO, Choi KW, Bellen HJ (1999) Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell 96: 833–845 [DOI] [PubMed] [Google Scholar]
- Bruckner K, Perez L, Clausen H, Cohen S (2000) Glycosyltransferase activity of Fringe modulates Notch–Delta interactions. Nature 406: 411–415 [DOI] [PubMed] [Google Scholar]
- de Celis JF, Bray S (1997) Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development 124: 3241–3251 [DOI] [PubMed] [Google Scholar]
- de Celis JF, Garcia Bellido A (1994) Roles of the Notch gene in Drosophila wing morphogenesis. Mech Dev 46: 109–122 [DOI] [PubMed] [Google Scholar]
- de Celis JF, Garcia-Bellido A, Bray SJ (1996) Activation and function of Notch at the dorsal–ventral boundary of the wing imaginal disc. Development 122: 359–369 [DOI] [PubMed] [Google Scholar]
- den Hollander AI (1999) Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat Genet 23: 217–221 [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea FJ, Cohen SM (1995) Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121: 4215–4225 [DOI] [PubMed] [Google Scholar]
- Doherty D, Fenger G, Younger-Shepherd S, Jan L-Y, Jan Y-N (1996) Dorsal and ventral cells respond differently to the Notch ligands Delta and Serrate during Drosophila wing development. Genes Dev 10: 421–434 [DOI] [PubMed] [Google Scholar]
- Fleming RJ, Gu Y, Hukriede NA (1997) Serrate-mediated activation of Notch is specifically blocked by the product of the gene fringe in the dorsal compartment of the Drosophila wing imaginal disc. Development 124: 2973–2981 [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, Perez L, Cohen SM (2002) A naturally occurring alternative product of the mastermind locus that represses notch signalling. Mech Dev 115: 101–105 [DOI] [PubMed] [Google Scholar]
- Guo M, Hong EJ, Fernandes J, Zipursky SL, Hay BA (2003) A reporter for amyloid precursor protein γ-secretase activity in Drosophila. Hum Mol Genet 12: 2669–2678 [DOI] [PubMed] [Google Scholar]
- Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW (2002) Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature 416: 178–183 [DOI] [PubMed] [Google Scholar]
- Lai EC (2004) Notch signaling: control of cell communication and cell fate. Development 131: 965–973 [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Rulifson EJ, Blair SS (1997) The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development 124: 1485–1495 [DOI] [PubMed] [Google Scholar]
- Moloney DJ (2000) Fringe is a glycosyltransferase that modifies Notch. Nature 406: 369–375 [DOI] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ (2000) Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102: 349–362 [DOI] [PubMed] [Google Scholar]
- Panin VM, Papayannopoulos V, Wilson R, Irvine KD (1997) Fringe modulates Notch–ligand interactions. Nature 387: 908–913 [DOI] [PubMed] [Google Scholar]
- Parks AL, Klueg KM, Stout JR, Muskavitch MA (2000) Ligand endocytosis drives receptor dissociation and activation in the notch pathway. Development 127: 1373–1385 [DOI] [PubMed] [Google Scholar]
- Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, Ready DF, Tepass U (2002) Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature 416: 143–149 [DOI] [PubMed] [Google Scholar]
- Struhl G, Adachi A (1998) Nuclear access and action of Notch in vivo. Cell 93: 649–660 [DOI] [PubMed] [Google Scholar]
- Struhl G, Adachi A (2000) Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol Cell 6: 625–636 [DOI] [PubMed] [Google Scholar]
- Sun X, Artavanis-Tsakonas S (1997) Secreted forms of DELTA and SERRATE define antagonists of Notch signaling in Drosophila. Development 124: 3439–3448 [DOI] [PubMed] [Google Scholar]
- Tepass U, Theres C, Knust E (1990) crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61: 787–799 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 1
Supplementary Material


