Abstract
The fibronectin-leucine-rich transmembrane (FLRT) family of leucine-rich repeat (LRR) proteins is implicated in fibroblast growth factor (FGF) signalling, early embryonic development and neurite outgrowth. Here, we have analysed whether FLRTs may also function in cell adhesion. We find that FLRT proteins can physically interact and that FLRT-transfected cultured cells sort out from non-transfected cells, suggesting a change in adhesive properties. A similar sorting effect is also observed in Xenopus embryos and tissue aggregates. FLRT-mediated cell sorting is calcium dependent and substrate independent. Deletion analysis indicates that cell sorting requires the LRR domains, which are dispensable for FLRT-mediated FGF signalling. Conversely, sorting is independent of the cytoplasmic domain, which is essential for FLRT-induced signalling. Therefore, FLRT-mediated FGF signal transduction and homotypic cell sorting can be molecularly uncoupled. The results indicate that FLRT proteins have a dual role, promoting FGF signalling and modulating homotypic cell adhesion.
Keywords: cell adhesion, FGF, FLRT, receptor tyrosine kinase
Introduction
Fibronectin-leucine-rich transmembrane (FLRT) proteins are a family of cell-surface molecules, which in adult human tissues are expressed in all organs tested (Lacy et al, 1999; Böttcher et al, 2004). FLRTs contain extracellular leucine-rich repeats (LRRs), a fibronectin type III (FNIII) domain and a cytoplasmic tail without obvious protein motifs. FLRT3, the best-characterized member of the family, is coexpressed with fibroblast growth factor (FGF) 8 and positively regulated by FGF signalling in Xenopus embryos. During early Xenopus development, FLRT3 functions as a positive modulator of FGF signalling (Böttcher et al, 2004). In gain- and loss-of-function experiments, FLRT3 and FLRT2 phenocopy FGF signalling. Xenopus FLRT3 physically interacts with FGF receptors (FGFRs) and by an unknown mechanism activates the mitogen-activated protein kinase cascade to promote FGF target gene expression. The ability of FLRT3 to activate FGF signalling is dependent on the cytoplasmic domain, and FGFR binding is mediated by the FNIII domain of FLRT3. In contrast, the LRR domains are dispensable for FGF signal activation (Böttcher et al, 2004).
In addition to regulating FGF signalling during embryonic development, FLRTs have also been implicated in neurite outgrowth. FLRT3 messenger RNA expression is upregulated after nerve injury (Tanabe et al, 2003; Robinson et al, 2004). The protein accumulates in damaged sensory neuron cell bodies and is exported into their peripheral and central processes (Robinson et al, 2004). Antisense knockdown of FLRT3 protein reduces whereas, overexpression of FLRT3 promotes neurite outgrowth from dorsal root ganglion neurons in vitro (Robinson et al, 2004), and an increase in neurite outgrowth is observed from neonatal cerebellar granule cells grown on Chinese hamster ovary (CHO) cells overexpressing FLRT3 (Tsuji et al, 2004).
The effect on neurite outgrowth together with the fact that some LRR proteins, such as chaoptin (Krantz & Zipursky, 1990), connectin (Nose et al, 1992), capricious and tartan (Milán et al, 2001), function in intercellular adhesion or tissue boundary formation raised the possibility that FLRTs may also have a role in cell adhesion. Here, we show that FLRT proteins can interact with each other and that they promote homotypic cell sorting.
Results
FLRT proteins physically interact
FLRTs were previously characterized as FGF pathway modulators, which interact physically and functionally with FGFRs (Böttcher et al, 2004). In addition to the FLRT–FGFR interaction, we also observed FLRT–FLRT interaction (Fig 1). In co-immunoprecipitation (co-IP), V5 epitope-tagged FLRT3 precipitates Flag epitope-tagged FLRT3 as well as FGFR1 (full-length and cytoplasmic domain-deleted (ΔC) protein), but not BMP type I receptor (BMPRI; Fig 1A). We confirmed this result by an alternative assay using V5-tagged FLRT3 to co-immunoprecipitate various Renilla luciferase (RL)-tagged proteins, which can be detected quantitatively in bioluminescence assays. RL–FLRT1, RL–FLRT2 and RL–FLRT3 were significantly enriched compared with unrelated transmembrane proteins (RL–BMPRI, RL–Frizzled8; Fig 1B). Finally, to test whether FLRTs can also interact in vivo, we carried out a bioluminescence resonance energy transfer (BRET) assay. RL–FLRT2 was coexpressed in human embryonic kidney 293T cells with various enhanced yellow fluorescent protein fusion proteins. As reported previously for FLRT3 (Böttcher et al, 2004), FLRT2 interacts with FGFR1ΔC but not with the full-length FGFR1, probably owing to steric reasons (Fig 1C). In addition, both FLRT1 and FLRT2 interact strongly with FLRT2. Homodimerization of secreted FLRT3 ectodomain (FLRT3ΔTM) in solution or binding of secreted FLRT3 to FLRT3-transfected cells was, however, not observed (data not shown). This may be because of inappropriate folding or post-translational modifications of the secreted form of FLRT3, but may also indicate that the observed FLRT–FLRT interaction depends on another binding partner.
Figure 1.
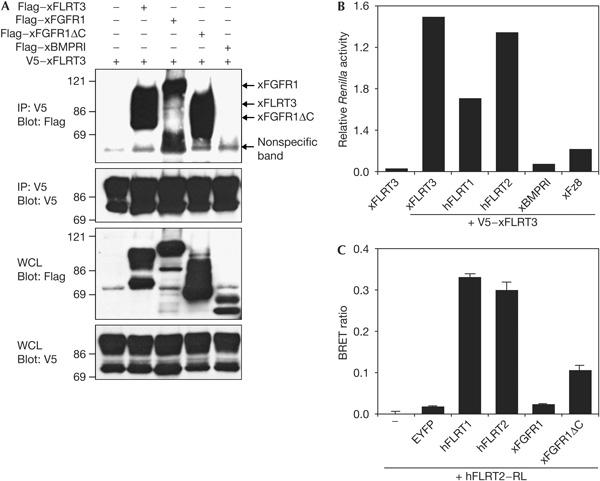
FLRT proteins interact physically with each other. (A) Human embryonic kidney 293T cells were co-transfected with the indicated tagged constructs and 48 h later were collected for a co-immunoprecipitation (co-IP)–western blot analysis. (B) Co-IP of 293T cells transfected with V5-tagged Xenopus FLRT3 and the indicated Renilla luciferase (RL)-tagged constructs. RL activity after co-IP was normalized against total RL activity of the lysates. (C) 293T cells were co-transfected with human FLRT2–RL fusion and the indicated enhanced yellow fluorescent protein (EYFP)-tagged constructs and analysed by bioluminescence resonance energy transfer (BRET) assay. Data are represented as BRET ratio, which is defined as E530/E470(sample)−E530/E470(hFLRT2–RL only). WCL, whole-cell lysate; xBMPRI, Xenopus BMP type I receptor; xFGFR1, Xenopus fibroblast growth factor receptor 1; xFz8, Xenopus Frizzled 8.
FLRT proteins promote homotypic cell sorting
While performing the above experiments, we noticed that FLRT overexpression in 293T cells gives rise to a characteristic cell-sorting behaviour. Within the confluent cell monolayer, the FLRT2- or FLRT3-transfected cells labelled by co-transfected enhanced green fluorescent protein (EGFP) segregate from the non-transfected, EGFP-negative cells (Fig 2A–C). Cell sorting is evident after 24 h and becomes fully developed 2–3 days after transfection. Intriguingly, the brightest cells usually sort to the interior of the transfected cell clusters, suggesting possible FLRT dose-dependent changes in cell–cell adhesion. FLRT-mediated sorting is independent of substrate adhesion, as it occurs when cells are seeded on agarose, where they form multilayered discoidal clusters (Fig 2D,E), or when aggregated in suspension (Fig 2F,G). Cell sorting is also observed under serum starvation (data not shown), suggesting that it is independent of cell proliferation. Mouse P19 cells also show FLRT-mediated sorting, but CHO, L cells, HeLa, Cos1 and RKO cells do not (supplementary Fig S1 online), indicating cell specificity of this effect. Interestingly, cell sorting by two types of cadherins was observed only in 293T cells (supplementary Fig S1 online), suggesting that this cell type is a particularly sensitive system for studying cell-sorting behaviour.
Figure 2.
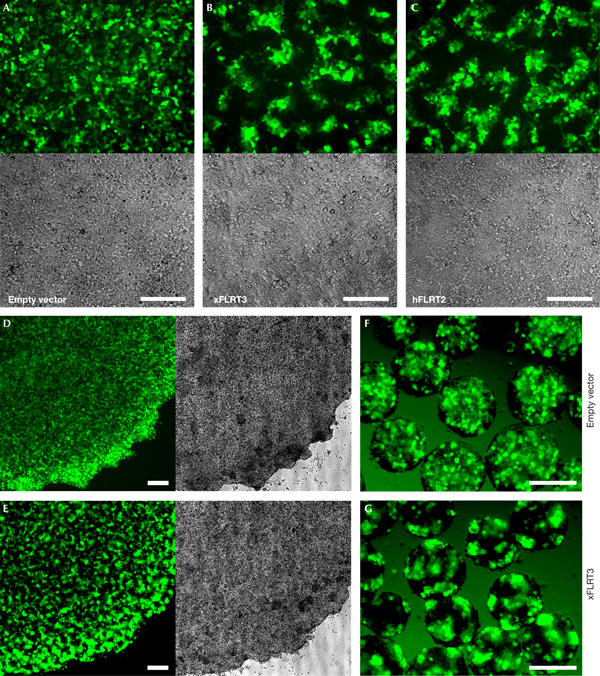
Overexpression of FLRT proteins causes cell sorting. (A–C) 293T cells were co-transfected with enhanced green fluorescent protein (EGFP) and Xenopus FLRT3 or human FLRT2 expression plasmids. Fluorescence and bright-field images of confluent monolayers were taken 60 h after transfection. (D–G) FLRT-mediated cell sorting does not require substrate adhesion. 24 h after transfection with Xenopus FLRT3 and EGFP, 293T cells were dissociated, mixed 1:1 with non-transfected cells, re-plated on agarose and photographed after 20 h (D,E) or aggregated in suspension and photographed after 48 h (F,G). Empty vector transfections served as sorting controls (A,D,F). Scale bars, 200 μm.
The LRR domains of FLRT3 are required for cell sorting
We next tested different FLRT3 deletion constructs in the cell-sorting assay (Fig 3). Secreted FLRT3 (FLRT3ΔTM) does not confer cell sorting (Fig 3B), suggesting that membrane localization is crucial for this activity. FLRT3ΔC lacking the intracellular domain is inactive in FGF signalling in Xenopus (Böttcher et al, 2004), but still induces cell sorting in 293T cells (Fig 3C). Likewise, removal of the FNIII domain, which mediates FGFR interaction (Böttcher et al, 2004), does not inhibit cell sorting (Fig 3D). In contrast, deletion of the LRR domains (FLRT3ΔLRR), which are dispensable for FLRT-mediated signalling, abolishes cell sorting (Fig 3E), even when overexpressed at levels much higher than those of the full-length protein (supplementary Fig S2 online). Transfection of plasmids encoding ten unrelated LRR transmembrane proteins in 293T cells did not result in cell-sorting behaviour (data not shown), implying that this phenomenon is not a general property of LRR transmembrane proteins. These data suggest that the role of FLRT3 in cell sorting is independent of its function in FGF signalling, and that the two functions can be molecularly uncoupled.
Figure 3.
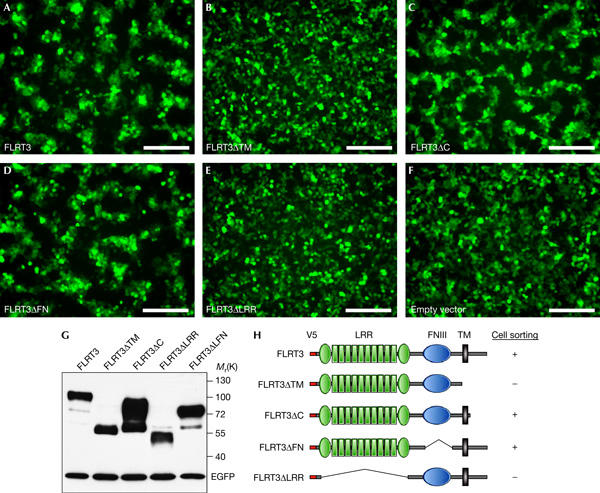
Domain analysis of FLRT3-mediated cell sorting. (A–F) 293T cells were co-transfected with enhanced green fluorescent protein (EGFP) and different V5-tagged Xenopus FLRT3 constructs. Fluorescence images of confluent cell monolayers were taken 65 h after transfection and, subsequently, the cells were collected for western blotting. Scale bars, 200 μm. (G) Protein production control by western blot analysis. (H) Summary of the results. FNIII, fibronectin type III domain; LRR, leucine-rich repeat; TM, transmembrane domain.
FLRT3-mediated cell sorting is calcium dependent
The experiments described above suggest that FLRT proteins may act as homophilic cell adhesion molecules or modulate cell–cell adhesion by interacting with other cell-surface proteins. Some intercellular adhesion molecules (cadherins, lectins) are known to be Ca2+ dependent, whereas others (for example, Ig-superfamily CAMs) do not require Ca2+ for binding. We therefore examined whether FLRT-mediated sorting of 293T cells is Ca2+ dependent (Fig 4). When cells are cultivated in a medium containing FCS as the only Ca2+ source, FLRT-mediated cell sorting is abolished. Addition of increasing amounts of Ca2+ causes dose-dependent cell sorting in the presence of FLRT3 in cells grown on plastic or agarose.
Figure 4.
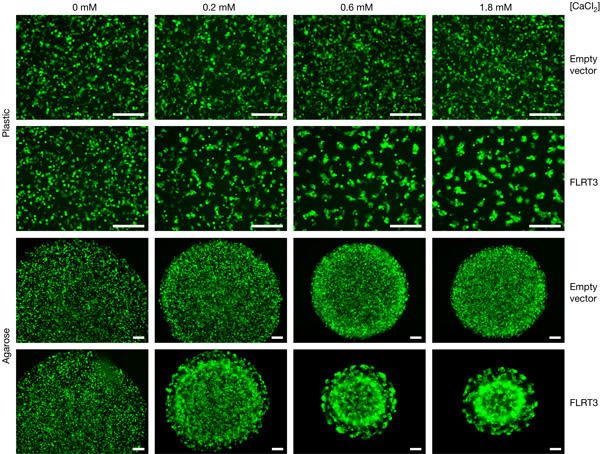
FLRT3-mediated cell sorting is calcium dependent. 293T cells were co-transfected with enhanced green fluorescent protein (EGFP) and either empty vector (control) or V5-tagged Xenopus FLRT3. 24 h after transfection, cells were dissociated, mixed 1:2 with non-transfected cells and re-plated on plastic or agarose in Ca2+-free DMEM supplemented with 10% FCS (a source of ∼ 0.2 mM Ca2+ final concentration) and the indicated final concentration of CaCl2. Pictures were taken after 24 h (plastic, confluent cells) or 68 h (agarose). Scale bars, 200 μm.
FLRT3ΔC promotes cell-sorting behaviour in vivo
We next tested FLRT3-mediated cell sorting in Xenopus embryos. When mRNA of FLRT3ΔC, which is inactive in FGF signalling and causes no morphological defects in Xenopus (Böttcher et al, 2004), is injected together with a lineage tracer (β-galactosidase) into one animal-ventral blastomere of 32-cell-stage Xenopus embryos, the normal cell mixing of the injected progeny with the surrounding cells during gastrulation is inhibited and the β-galactosidase-positive cells remain more compact (Fig 5C,E). Similarly, an animal cap dissociation and co-aggregation assay showed that embryonic cells expressing FLRT3ΔC sort out in discrete patches, which tend to localize preferentially on the surface of the tissue aggregates (Fig 5D,F). Similar cell-sorting behaviour and inhibition of cell mixing in vivo were previously reported for the homophilic cell adhesion molecule paraxial protocadherin (Kim et al, 1998).
Figure 5.
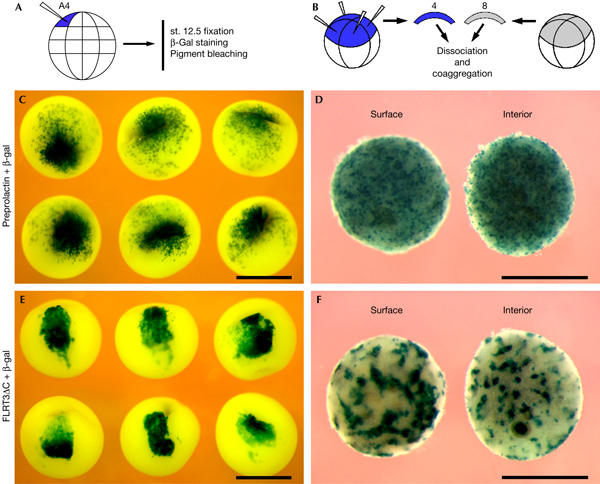
FLRT3ΔC promotes cell-sorting behaviour in vivo. (A,B) Schemes of the experiments. (C,E) Ectodermal cell mingling is inhibited by FLRT3ΔC. Xenopus embryos at 32-cell-stage were injected in one animal-ventral (A4) blastomere with β-galactosidase (β-gal) mRNA (75 pg) together with either control (preprolactin) or FLRT3ΔC mRNA (300 pg), fixed at late gastrula stage 12.5, β-gal stained and bleached of pigment. (D,F) Sorting out of FLRT3ΔC-expressing embryonic cells. Animal cap explants from embryos that were injected with either preprolactin/β-gal or FLRT3ΔC/β-gal mRNA and from embryos that were not injected were mixed (four and eight caps, respectively), dissociated, co-aggregated, bisected and stained for β-gal activity. Surface and interior views of bisected aggregates are shown. Scale bars, 1 mm.
Discussion
Our data showing that FLRTs promote homotypic cell sorting suggest a role for these cell-surface molecules in cell adhesion. One obvious possibility is that FLRTs act as homophilic cell adhesion molecules. This conclusion is supported by the finding that FLRT proteins can interact with each other, although it is unclear at present whether this interaction is direct. In addition, the possibility remains that FLRTs may interact with other cell-surface molecules in a heterophilic manner to modulate homotypic cell adhesion. This may explain the absence of homophilic binding of secreted forms of FLRT3 in solution, as well as the reported lack of change in the aggregation rate of FLRT3-expressing CHO cells (Robinson et al, 2004).
The fact that FLRT-mediated cell sorting is dependent on the LRR domains (which are dispensable for FGF signalling) but independent of its cytoplasmic domain (required for FGF signalling) rules out an indirect effect through FGF signal activation. The dual role of FLRTs in cell adhesion and FGF signalling is reminiscent of the common theme, where cell adhesion molecules not only mediate cell–cell interactions but also regulate receptor tyrosine kinases (Orian-Rousseau et al, 2002; Suyama et al, 2002; Birchmeier et al, 2003; Christofori, 2003; Ponta et al, 2003; Cavallaro & Christofori, 2004). In the case of FGF signalling, the homophilic cell adhesion molecules N-cadherin and N-CAM physically interact with FGFRs and promote their signal transduction (Cavallaro et al, 2001; Williams et al, 2001; Suyama et al, 2002). Interestingly, the interaction with FGFR1 involves the FNIII domain of N-CAM (Kiselyov et al, 2003), as is the case for FLRT3 (Böttcher et al, 2004). Thus, FLRTs may represent yet another family of cell-surface proteins that link cell adhesion and signal transduction (Fig 6).
Figure 6.
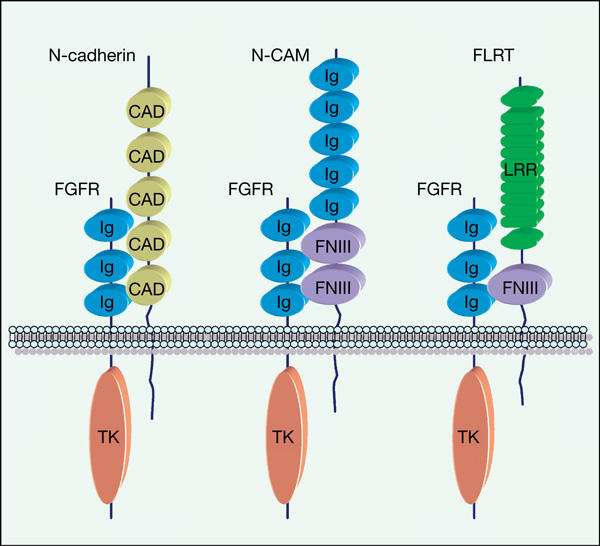
Schematic drawing of cell adhesion molecules interacting with fibroblast growth factor receptors (FGFRs). CAD, cadherin repeats; FNIII, fibronectin type III domain; Ig, immunoglobulin domain; LRR, leucine-rich repeat; TK, tyrosine kinase domain.
The LRR is a widespread protein motif, which is found in a diverse set of intracellular, membrane-associated and extracellular proteins from bacteria to vertebrates and is frequently implicated in homo- and heterophilic protein–protein interactions (Buchanan & Gay, 1996; Kobe & Kajava, 2001). However, despite the large number of LRR proteins and the sequence similarity among them within the LRR domain, they are highly specific with regard to their binding partners (Fournier et al, 2001; Lin et al, 2003). Several cell-surface LRR proteins, such as connectin, chaoptin, capricious and tartan (Krantz & Zipursky, 1990; Nose et al, 1992; Milán et al, 2001), are implicated in intercellular adhesion, cell sorting or boundary formation during development. Our study provides evidence that FLRTs might modulate, directly or indirectly, cell–cell adhesion through their extracellular LRR domains. Deregulation of cell adhesion is a hallmark of many cancers, and therefore it will be interesting to see whether FLRT proteins are involved in tumorigenesis. It is noteworthy that FLRT2 expression is strongly downregulated in certain ovarian and uterine cancers (Donninger et al, 2004; Santin et al, 2004, 2005).
Methods
Cell culture, transfection and microscopy. 293T cells were grown in DMEM media supplemented with 10% FCS, 2 mM L-glutamine and penicillin/streptomycin in a humidified incubator with 5% CO2. Transient transfections with FuGENE 6 (Roche, Mannheim, Germany) were carried out on 50–70% confluent cells with 150–300 ng pCS-FLRTs (Böttcher et al, 2004) and 10–20 ng pCS-EGFP per well of a 24-well plate. Typically, between 40% and 60% of the cells were transfected, as judged by EGFP fluorescence. In some experiments, cells were briefly trypsinized 24 h after transfection, mixed with non-transfected cells and re-plated in fresh medium in 24- or 96-well plates coated with a thin layer of 1% agarose, or aggregated in suspension culture with orbital shaking (120 r.p.m.) in BSA-coated 24-well plates. Photographs were taken with an inverted fluorescent microscope (Nikon) connected to a cooled monochrome CCD camera (Photometrics) and subsequently pseudocoloured in green.
Co-immunoprecipitation, western blotting and BRET assays. 293T cells were lysed in NOP buffer (20 mM Tris, 150 mM NaCl, 2% Nonidet P-40, 1 mM EDTA and protease inhibitors) on ice for 10–20 min. Lysates were cleared by centrifugation, the primary antibody was added to the supernatant and the solution was incubated for 1–4 h at 4°C with rotation. After the addition of anti-mouse IgG agarose beads (Sigma, Munich, Germany), samples were rotated overnight at 4°C. After washing five times with immunoprecipitation buffer (20 mM Na2HPO4, 0.5 M NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate (pH 7.5) and 0.02% NaN3), precipitates were separated by 7.5% SDS–polyacrylamide gel electrophoresis and immunoblotted with the appropriate antibodies. For co-IP of RL fusion proteins, 293T cells were transfected in 24-well plates in triplicate. 48 h after transfection, the triplicates were pooled and the cells lysed with 500 μl NOP buffer. Then, 10% of the lysate was transferred to 96-well luminometer plates to determine the RL activity of the lysate before co-IP. Anti-V5 antibody (1 μl; Invitrogen, Karlsruhe, Germany) was added to the remaining lysate and incubated for 1 h on ice. 12 μl of anti-mouse IgG agarose beads was added and the lysate rotated overnight at 4°C. After washing five times with immunoprecipitation buffer, precipitates were resuspended in 60 μl PBS and transferred to 96-well luminometer plates. RL activity was measured using an Ascent FL luminometer (Labsystems, Helsinki, Finland) after the addition of coelenterazine h (Molecular Probes, Eugene, OR, USA) to a final concentration of 5 μM. Relative RL activity of individual samples after co-IP was determined by normalizing to the total activity of the respective lysate. BRET assays were carried out as described (Böttcher et al, 2004).
Embryos and explants. Xenopus embryo staging, microinjection, explantation, fixation and pigment bleaching, mRNA synthesis and β-galactosidase staining were carried out according to standard protocols (Sive et al, 2000). For the animal cap dissociation and co-aggregation assay, eight-cell-stage embryos were microinjected in each animal blastomere with 1 ng of FLRT3ΔC or control (preprolactin) mRNA together with 200 pg β-galactosidase mRNA as a lineage tracer. Animal cap explants, excised at the late blastula stage from injected and non-injected embryos, were mixed (1:2 ratio, triplicate samples) and dissociated in Ca2+/Mg2+-free medium. The outer (epithelial) layer of the explants, which remained intact under these conditions, was removed and the inner (sensorial) layer was fully dispersed by pipetting and aggregated for 18 h in agarose-coated 24-well plates in 0.6 × MMR medium, then fixed, bisected, stained for β-galactosidase activity and bleached of the residual pigment. Similar results with three batches of embryos were obtained.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400614-s1.pdf).
Supplementary Material
Supplementary Information
Acknowledgments
We thank P. Stannek and U. Fenger for excellent technical help, D. Wedlich, L. Kunkel and B. Mao for plasmids, W. Wu and A. Glinka for helpful discussions and G. Davidson for critically reading the manuscript and for useful comments. This work was supported by the Wilhelm Sander Stiftung.
References
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4: 915–925 [DOI] [PubMed] [Google Scholar]
- Böttcher RT, Pollet N, Delius H, Niehrs C (2004) The transmembrane protein XFLRT3 forms a complex with FGF receptors and promotes FGF signalling. Nat Cell Biol 6: 38–44 [DOI] [PubMed] [Google Scholar]
- Buchanan SG, Gay NJ (1996) Structural and functional diversity in the leucine-rich repeat family of proteins. Prog Biophys Mol Biol 65: 1–44 [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G (2004) Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 4: 118–132 [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Niedermeyer J, Fuxa M, Christofori G (2001) N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol 3: 650–657 [DOI] [PubMed] [Google Scholar]
- Christofori G (2003) Changing neighbours, changing behaviour: cell adhesion molecule-mediated signalling during tumour progression. EMBO J 22: 2318–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donninger H, Bonome T, Radonovich M, Pise-Masison CA, Brady J, Shih JH, Barrett JC, Birrer MJ (2004) Whole genome expression profiling of advance stage papillary serous ovarian cancer reveals activated pathways. Oncogene 23: 8065–8077 [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM (2001) Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409: 341–346 [DOI] [PubMed] [Google Scholar]
- Kim SH, Yamamoto A, Bouwmeester T, Agius E, De Robertis EM (1998) The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development 125: 4681–4690 [DOI] [PubMed] [Google Scholar]
- Kiselyov VV et al. (2003) Structural basis for a direct interaction between FGFR1 and NCAM and evidence for a regulatory role of ATP. Structure (Camb) 11: 691–701 [DOI] [PubMed] [Google Scholar]
- Kobe B, Kajava AV (2001) The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol 11: 725–732 [DOI] [PubMed] [Google Scholar]
- Krantz DE, Zipursky SL (1990) Drosophila chaoptin, a member of the leucine-rich repeat family, is a photoreceptor cell-specific adhesion molecule. EMBO J 9: 1969–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy SE, Bönnemann CG, Buzney EA, Kunkel LM (1999) Identification of FLRT1, FLRT2, and FLRT3: a novel family of transmembrane leucine-rich repeat proteins. Genomics 62: 417–426 [DOI] [PubMed] [Google Scholar]
- Lin JC, Ho WH, Gurney A, Rosenthal A (2003) The netrin-G1 ligand NGL-1 promotes the outgrowth of thalamocortical axons. Nat Neurosci 6: 1270–1276 [DOI] [PubMed] [Google Scholar]
- Milán M, Weihe U, Pérez L, Cohen SM (2001) The LRR proteins capricious and Tartan mediate cell interactions during DV boundary formation in the Drosophila wing. Cell 106: 785–794 [DOI] [PubMed] [Google Scholar]
- Nose A, Mahajan VB, Goodman CS (1992) Connectin: a homophilic cell adhesion molecule expressed on a subset of muscles and the motoneurons that innervate them in Drosophila. Cell 70: 553–567 [DOI] [PubMed] [Google Scholar]
- Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H (2002) CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev 16: 3074–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta H, Sherman L, Herrlich PA (2003) CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4: 33–45 [DOI] [PubMed] [Google Scholar]
- Robinson M et al. (2004) FLRT3 is expressed in sensory neurons after peripheral nerve injury and regulates neurite outgrowth. Mol Cell Neurosci 27: 202–214 [DOI] [PubMed] [Google Scholar]
- Santin AD et al. (2004) Gene expression profiles in primary ovarian serous papillary tumors and normal ovarian epithelium: identification of candidate molecular markers for ovarian cancer diagnosis and therapy. Int J Cancer 112: 14–25 [DOI] [PubMed] [Google Scholar]
- Santin AD et al. (2005) Gene expression fingerprint of uterine serous papillary carcinoma: identification of novel molecular markers for uterine serous cancer diagnosis and therapy. Br J Cancer 92: 1561–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM (2000) Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Suyama K, Shapiro I, Guttman M, Hazan RB (2002) A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2: 301–314 [DOI] [PubMed] [Google Scholar]
- Tanabe K, Bonilla I, Winkles JA, Strittmatter SM (2003) Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J Neurosci 23: 9675–9686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji L, Yamashita T, Kubo T, Madura T, Tanaka H, Hosokawa K, Tohyama M (2004) FLRT3, a cell surface molecule containing LRR repeats and a FNIII domain, promotes neurite outgrowth. Biochem Biophys Res Commun 313: 1086–1091 [DOI] [PubMed] [Google Scholar]
- Williams EJ, Williams G, Howell FV, Skaper SD, Walsh FS, Doherty P (2001) Identification of an N-cadherin motif that can interact with the fibroblast growth factor receptor and is required for axonal growth. J Biol Chem 276: 43879–43886 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


