The generation of proteomic diversity from a limited number of genes is a problem faced by many higher organisms with complex tissues. Alternative splicing is one well-established solution to this problem. It has been reported that more than 70% of all human genes are alternatively spliced (Johnson et al, 2003). Another method to increase proteomic diversity is RNA editing. In animals, adenosine deaminases that act on RNA (ADARs) convert adenosines to inosines in structured or double-stranded RNAs (dsRNAs). As inosines are interpreted as guanosines by the ribosome, A to I editing is the functional equivalent of an A to G change and can alter the coding potential of an RNA. The introduction of an inosine can also generate or delete splice sites, and therefore has a twofold impact on RNA diversity.
Editing levels are highest in the brain, possibly reflecting the need for increased proteomic variation in this tissue. Well-studied editing substrates in brain tissue include subunits of the glutamate ion channel family or the serotonin 5HT-2c receptor. In both substrates, editing alters the coding potential of the RNA, which leads to the formation of proteins with altered properties. Interestingly, in these and many other substrates, the dsRNA structure required for editing is formed by base pairing of intronic and exonic sequences. Editing is therefore generally thought to be a co-transcriptional process that occurs before the removal of introns. Accordingly, the speed of splicing would regulate the availability of binding sites for ADARs and thus the extent of editing.
However, in ADAR2-knockout animals, editing of the RNA that encodes glutamate receptor B (GluR-B) reveals a different picture (Higuchi et al, 2000). The absence of editing prevents splicing of an intron adjacent to the so-called Q/R editing site, which shows that editing can regulate splicing. Such a regulatory loop could lead to nuclear retention of unedited and therefore unspliced transcripts, and would control the level of edited transcripts in the cytoplasm. In fact, about 99% of all glutamate receptor B subunits are from the edited version; even a slight increase in unedited transcripts is detrimental to life (Brusa et al, 1995). The proximity of regions of alternative splicing and editing in the GluR-B and 5HT-2c transcripts provide an additional link between these two processes (Lomeli et al, 1994; Wang et al, 2000). Several reports indicate that splice-site choice might be regulated by editing (Agrawal & Stormo, 2005; Maas et al, 2001).
Now, another piece has been added to this stunning puzzle that ultimately leads to a diversification of the transcriptome. In this issue of EMBO reports, Laurencikiene and colleagues report an editing event that is dependent on the C-terminal domain (CTD) of RNA polymerase II (pol II; Laurencikiene et al, 2006). The CTD is generally believed to couple transcription to RNA-processing events. It forms a tail-like extension from the catalytic core of pol II and consists of heptapeptide repeats that vary in number between species. Changes in phosphorylation produce different states of the polymerase. Whereas an unphosphorylated CTD is required for the initiation of transcription, phosphorylated CTD is the elongation-competent form and associates with many mRNA-processing factors involved in capping, splicing and polyadenylation. A close connection between mRNA splicing and transcription has been suggested and, in a few specific cases, a direct biochemical link has been made. Protein factors with homology to Ser–Arg-rich (SR) proteins and Prp40—a component of the yeast U1 small nuclear ribonucleoprotein particle (snRNP)—are known to interact directly with phospho-CTD (Corden & Patturajan, 1997; Morris & Greenleaf, 2000).
Although there is still much to learn about the precise role of the CTD in co-transcriptional processing, it seems safe to say that it facilitates the assembly of the processing machinery on the nascent transcript, thereby coordinating the above-mentioned RNA processing events. This recent report by Laurencikiene and colleagues adds RNA editing to the list of RNA-processing events that are coordinated by the CTD (Fig 1).
Figure 1.
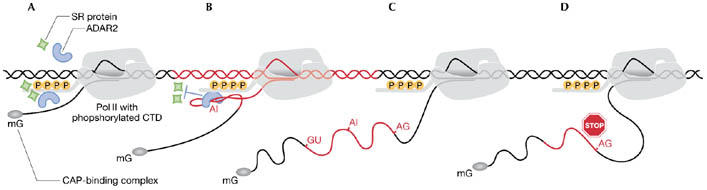
Regulation of editing by the C-terminal domain of RNA polymerase II. During transcription, the C-terminal domain (CTD) of polymerase II (pol II) is hyperphosphorylated (A) and serves as a platform for the assembly and recruitment of RNA processing factors. (B) During autoediting of the adenosine deaminases that act on RNA 2 (ADAR2) pre-mRNA, the CTD facilitates editing, possibly by selectively binding ADAR2 and blocking access of splicing factors—therefore preventing premature splicing of the ADAR2 pre-mRNA. (C) Editing of the ADAR2 pre-mRNA generates an extra out-of-frame splice site. (D) Use of this new site introduces a premature stop-codon to the spliced message and leads to the synthesis of a catalytically inactive ADAR2 peptide.
In their study, the authors take advantage of an RNA-editing event in the ADAR2 pre-mRNA. This auto-editing event in intron 4 creates a new splice acceptor (Rueter et al, 1999). Its use results in the inclusion of an additional 47 nucleotides, generating a −1 frame shift that produces a truncated, catalytically inactive version of the protein. This regulation of alternative splicing by editing provides an autoregulatory feedback loop that allows ADAR2 to modulate its own activity (Feng et al, 2006; Rueter et al, 1999).
The authors show that autoediting and subsequent alternative splicing of the ADAR2 pre-mRNA site is dependent on the presence of ADAR2 and is strongly stimulated by the presence of the CTD (Laurencikiene et al, 2006). Pol II was used, with or without the CTD, to drive the expression of a reporter construct containing the editing site of intron 4 of ADAR2. At the same time, endogenous pol II was inhibited by α-amanitin. Editing levels were then measured in terms of the amount of alternative splicing. Interestingly, the level of alternatively spliced transcripts was significantly reduced when expression was driven by a pol II lacking the CTD, indicating that either editing or alternative splicing was inhibited by deletion of the CTD.
The use of a pre-edited reporter construct allowed the authors to distinguish alternative splicing from editing. Most of the pre-edited transcripts were shown to be alternatively spliced, regardless of whether the CTD was deleted. This indicates that splice-site selection was not affected by the CTD. Partial deletions of the CTD showed that either heptads 1–25 or heptads 27–52 are sufficient to support efficient editing of the ADAR2 pre-mRNA. Although these data cannot completely rule out a role for the CTD in recognizing the atypical inosine-containing splice site, the efficiency of RNA editing most likely depends on the CTD of pol II.
Laurencikiene and colleagues show that splicing efficiency at the ADAR2 editing site is not influenced by the presence of the CTD. This is surprising, as the CTD has been implicated as a key factor in the assembly and delivery of the splicing machinery to newly transcribed RNAs in vivo. In a previous paper from the same group, it was reported that editing and splicing compete with each other in vitro, but seem to be coordinated during transcription, possibly through pol II (Bratt & Öhman, 2003).
When combined with the fact that some splicing events are clearly dependent on editing, the current findings strongly argue for a regulatory role of the CTD in the coordination of splicing and editing. It remains to be determined whether this coordination requires direct or indirect interactions with the ADAR2 protein and whether such an interaction is in competition with the binding of SR proteins.
RNA-processing events such as capping, splicing and cleavage/ polyadenylation can influence and sometimes depend on each other. These findings indicate that editing should be added as a fourth player to the game, with the CTD playing the referee in the field.

References
- Agrawal R, Stormo GD (2005) Editing efficiency of a Drosophila gene correlates with a distant splice site selection. RNA 11: 563–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt E, Öhman M (2003) Coordination of editing and splicing of glutamate receptor pre-mRNA. RNA 9: 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, Seeburg PH, Sprengel R (1995) Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science 270: 1677–1680 [DOI] [PubMed] [Google Scholar]
- Corden JL, Patturajan M (1997) A CTD function linking transcription to splicing. Trends Biochem Sci 22: 413–416 [DOI] [PubMed] [Google Scholar]
- Feng Y, Sansam CL, Singh M, Emeson RB (2006) Altered RNA editing in mice lacking ADAR2 autoregulation. Mol Cell Biol 26: 480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH (2000) Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature 406: 78–81 [DOI] [PubMed] [Google Scholar]
- Johnson J, Castle J, Garrett-Engele P, Kan Z, Loerch P, Armour C, Santos R, Schadt E, Stoughton R, Shoemaker D (2003) Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science 302: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Laurencikiene J, Källman AM, Fong N, Bentley DL, Öhman M (2006) RNA editing and alternative splicing: the importance of co-transcriptional coordination. EMBO Rep 7: 303–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH (1994) Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science 266: 1709–1713 [DOI] [PubMed] [Google Scholar]
- Maas S, Patt S, Schrey M, Rich A (2001) Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proc Natl Acad Sci USA 98: 14687–14692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DP, Greenleaf AL (2000) The splicing factor, Prp40, binds the phosphorylated carboxyl-terminal domain of RNA polymerase II. J Biol Chem 275: 39935–39943 [DOI] [PubMed] [Google Scholar]
- Rueter SM, Dawson TR, Emeson RB (1999) Regulation of alternative splicing by RNA editing. Nature 399: 75–79 [DOI] [PubMed] [Google Scholar]
- Wang Q, O'Brien PJ, Chen CX, Cho DS, Murray JM, Nishikura K (2000) Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin2C receptors. J Neurochem 74: 1290–1300 [DOI] [PubMed] [Google Scholar]


