Abstract
The ability of psychrophiles to survive and proliferate at low temperatures implies that they have overcome key barriers inherent to permanently cold environments. These challenges include: reduced enzyme activity; decreased membrane fluidity; altered transport of nutrients and waste products; decreased rates of transcription, translation and cell division; protein cold-denaturation; inappropriate protein folding; and intracellular ice formation. Cold-adapted organisms have successfully evolved features, genotypic and/or phenotypic, to surmount the negative effects of low temperatures and to enable growth in these extreme environments. In this review, we discuss the current knowledge of these adaptations as gained from extensive biochemical and biophysical studies and also from genomics and proteomics.
Keywords: cold adaptation, enzymes, genomics, membrane fluidity, psychrophiles
Introduction
Psychrophilic microorganisms have successfully colonized all permanently cold environments from the deep sea to mountain and polar regions. Some of these organisms, depending on their optimal growth temperature, are also known by the terms psychrotolerant or psychrotroph (Morita, 1975). Nevertheless, we believe that there is a continuum in temperature adaptation for life with wide or narrow growth temperature ranges depending on the microorganism, and we will use the general term psychrophiles in this review to designate all microorganisms growing well at temperatures around the freezing point of water. This unique property implies that psychrophiles have successfully overcome two main challenges: first, low temperature, because any decrease in temperature exponentially affects the rate of biochemical reactions; and second, the viscosity of aqueous environments, which increases by a factor higher than two between 37 °C and 0 °C. Remarkable adaptations have been observed with some organisms such as Moritella profunda, which is a psychropiezophilic organism—a microorganism adapted to cold and living in the deep sea—that shows maximal growth rates at 2 °C and a maximum growth temperature of only 12 °C (Xu et al, 2003). This indicates that at temperatures as low as 2 °C, some enzymes or supramolecular structures already show an altered conformation that negatively affects the metabolic flux.
The aim of this review is to summarize what we know about the cold adaptation of psychrophilic microorganisms. Obvious targets of the deleterious effects of low temperatures are cytoplasmic membranes and enzymes that tend to rigidify when the temperature drops. This affects membrane permeability, and hence the transport of nutrients and waste products, and catalysis, because enzymes require a certain flexibility to function (Goodchild et al, 2004b; Ratkowsky et al, 2005). Impaired protein folding and protein cold-denaturation can also cause problems at low temperatures, in particular for bacterial strains that sustain biological activities at temperatures as low as −20 °C and resist freezing. Cold-shock proteins have also been described. What are their roles in this adaptation? Key biological activities that involve nucleic acids—such as DNA replication, transcription and translation—can also suffer from exposure to low temperatures through the formation of secondary structures or super-coiled structures; how do psychrophiles cope with these phenomena? Finally, several genome sequences of psychrophilic microorganisms have been determined, and partial annotation of these has revealed unpredicted cold adaptations, the number of which will obviously expand after completion of the analysis and genome sequencing of other psychrophiles.
Biodiversity
The lowest temperature limit for life seems to be around −20 °C, which is the value reported for bacteria living in permafrost soil and in sea ice. Microbial activity at such temperatures is restricted to small amounts of unfrozen water inside the permafrost soil or the ice, and to brine channels. These contain high concentrations of salts, exopolymeric substances and/or particulate matter, and fluid flow is maintained by concentration and temperature gradients. Aerobic and anaerobic bacteria are found at these temperatures. Other factors such as osmotic and hydrostatic pressure, solar, earth and cosmic radiation, oxidative stress and nutrient availability also strongly affect living conditions. Consequently, adaptation to cold is often combined with other adaptations. Despite all of these challenges, life thrives in these environments with a remarkable microbial biodiversity of mainly bacteria, fungi (in particular yeasts) and microalgae. Among the bacteria that have been detected, the most commonly reported microorganisms are the Gram-negative α-, β- and γ-proteobacteria (Pseudomonas spp. and Vibrio spp.) and the Cytophaga–Flavobacterium–Bacteriodes phylum. Coryneforms, Arthrobacter sp. and Micrococcus sp. are the most frequently found Gram-positive bacteria. Bacteria generally dominate in number and diversity over Archaea, although in some areas such as deep-sea waters, these are found in equivalent numbers, with Methanogenium and Methanococcus being the most cited genera. Among identified cyanobacteria, Oscillatoria, Phormidium and Nostoc commune are dominant in most of the Antarctic habitats (Pandey et al, 2004). Psychrophilic yeasts, particularly Cryptococcus spp., have been isolated repeatedly from soil samples and some researchers have described them as the most important life form in Antarctic desert soils (Vishniac & Klinger, 1986). Refer to Deming (2002) for a detailed description of the types of community associated with specific cold environments.
Membrane fluidity
Decreasing temperatures have an adverse effect on the physical properties and functions of membranes, typically leading to a reduction in membrane fluidity, the onset of a gel-phase transition and, ultimately, a loss of function. The lipid composition governs the physical properties of membranes and hence it is not surprising that this varies with the thermal habitat of the microorganism. In general, lower growth temperatures produce a higher content of unsaturated, polyunsaturated and methyl-branched fatty acids, and/or a shorter acyl-chain length, with studies reporting a high proportion of cis-unsaturated double-bonds and antesio-branched fatty acids (Chintalapati et al, 2004; Russell, 1997). This altered composition is thought to have a key role in increasing membrane fluidity by introducing steric constraints that change the packing order or reduce the number of interactions in the membrane. Further adaptations that have been suggested to increase membrane fluidity include an increased content of large lipid head groups, proteins and non-polar carotenoid pigments (Chintalapati et al, 2004). However, these adaptive strategies do not seem to be widespread, and studies show more compact lipid head groups (Arthur & Watson, 1976) and decreased non-polar carotenoid pigment synthesis (Fong et al, 2001) in some psychrophiles.
Transcription and translation
Some of the main barriers to protein synthesis at low temperatures include: reduced activity of transcriptional and translational enzymes; reduced protein folding, owing primarily to a reduced rate of prolyl isomerization; and a stabilization of DNA and RNA secondary structures. In psychrophiles, enzymes involved in these processes have adapted to be optimally active at low temperatures. For example, a ribosomal extract, RNA polymerase, elongation factor and peptidyl–prolyl cis–trans isomerase have all been shown to retain activity near 0 °C in several psychrophilic microorganisms. Indeed, this latter enzyme catalyses cis–trans prolyl isomerizations, and its high activity and overexpression at low temperatures might be important for maintaining protein-folding rates at low temperatures. Furthermore, nucleic-acid-binding proteins—for example, Escherichia coli CspA-related proteins—and RNA helicases that might be important for the destabilization of DNA and RNA secondary structures are also overexpressed at low temperatures in psychrophiles (Berger et al, 1996; Lim et al, 2000).
Cold-shock and heat-shock responses
The exposure of mesophilic organisms to sudden temperature changes, both upshifts and downshifts, induces the transient overexpression of several proteins—known respectively as heat-shock proteins (Hsps) or cold-shock proteins (Csps)—that are involved in various cellular processes such as transcription, translation, protein folding and the regulation of membrane fluidity (Phadtare, 2004). Although studies of these responses in psychrophilic microorganisms are still in their infancy, similarities with the Csps and Hsps that are induced in mesophiles have been observed. In particular, increased levels of nucleic-acid-binding proteins (for example, CspA-related proteins; Inouye & Phadtare, 2004) and chaperones, such as GroEL (Tosco et al, 2003) and DnaK (Yoshimune et al, 2005), have been frequently reported. However, distinctions do exist between the mesophilic and psychrophilic cold-shock response, including the lack of repression of housekeeping protein synthesis and the presence of cold-acclimation proteins (Caps) in psychrophiles. Many of the Csps observed in mesophiles act as Caps in psychrophiles, being constitutively rather than transiently expressed at low temperatures. Furthermore, this differential regulation of expression indicates that a temperature sensory system exists in psychrophiles, and thermosensors at the cell membrane level—that sense changes in fluidity—have been reported (Ray et al, 1994).
Antifreeze proteins and cryoprotectants
Antifreeze proteins (AFPs) have the ability to bind to ice crystals through a large complementary surface and thereby create thermal hysteresis and lower the temperature at which an organism can grow (Jia & Davies, 2002). AFPs have been recently demonstrated in Antarctic lake bacteria (Gilbert et al, 2004), one of which, from Marinomonas primoryensis, is Ca2+-dependent and hyperactive (Gilbert et al, 2005). The AFP from the Arctic plant growth-promoting rhizobacterium Pseudomonas putida GR12-2 shows both antifreeze and ice-nucleating activities (Muryoi et al, 2004).
Trehalose and exopolysaccharides (EPSs) might also have an important role in cryoprotection in psychrophiles. Trehalose is thought to have a colligative effect, but probably also helps in preventing protein denaturation and aggregation (Phadtare, 2004). Conversely, high concentrations of EPSs have been found in Antarctic marine bacteria (Nichols et al, 2005) and in Arctic winter sea ice (Krembs et al, 2002) These modify the physico-chemical environment of bacterial cells, participate in cell adhesion to surfaces and retention of water, favour the sequestration and concentration of nutrients, retain and protect extracellular enzymes against cold denaturation and also act as cyoprotectants (Mancuso Nichols et al, 2005).
Cold-adapted enzymes
The most important selective pressure of low temperatures is exerted towards chemical reaction rates, most of which exponentially drop with decreasing temperature according to:
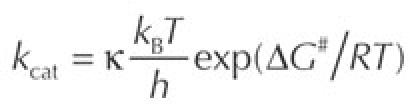 |
in which kcat is the reaction rate, κ is the transmission coefficient, kB is the Boltzmann constant, T is the absolute temperature in Kelvin, h is the Planck constant, R is the gas constant and ΔG# is the activation energy. κ is generally considered to be equal or close to one; however, this transmission coefficient significantly varies with viscosity, resulting in a further decrease of kcat (Siddiqui et al, 2004). Despite this, psychrophiles produce cold-adapted enzymes that have high specific activities at low temperatures (Fig 1), often up to an order of magnitude higher than those observed for their mesophilic counterparts (Feller & Gerday, 2003; Georlette et al, 2004; Russell, 2000). The commonly accepted hypothesis for this cold adaptation is the activity–stability–flexibility relationship, which suggests that psychrophilic enzymes increase the flexibility of their structure to compensate for the ‘freezing effect' of cold habitats (Johns & Somero, 2004). This increased flexibility might concern the entire protein or might be restricted to parts of the structure, especially those implicated in catalysis, and is probably also responsible for the generally observed low stability of cold-adapted proteins (Collins et al, 2003; D'Amico et al, 2003). Conversely, it has been shown that activity and stability are apparently not always inversely linked (Wintrode et al, 2001). However, in this study, multisubstrate enzymes and small-size synthetic substrates were mainly used, which might produce different results to those obtained with natural, large substrates; that is, the specificity of the enzyme might simply be shifted towards the substrate used.
Figure 1.
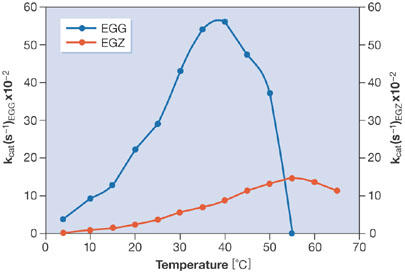
Thermodependence of activity for the cold-adapted cellulase from Pseudoalteromonas haloplanktis (EGG) and its mesophilic homologue from Erwinia chrysanthemi (EGZ).
Crystallographic structures of psychrophilic proteins indicate that these do not have unusual conformations but instead share a high similarity with their meso- and thermophilic homologues. To enhance flexibility, many structural modifications that lead to attenuation in strength and/or number of stabilizing factors—enthalpic or entropic—have been observed. Common trends include: the reduction of the number of ion pairs, hydrogen bonds and hydrophobic interactions; decreased intersubunit interactions; increased interaction with the solvent; a reduced apolar fraction in the core; higher accessibility to the active site; increased exposure of apolar residues to the solvent; decreased cofactor binding; clustering of glycine residues; and a lower proline and arginine content (see Violot et al, 2005).
Comparison of the thermodynamic parameters of activation of psychrophilic enzymes with those of their mesophilic homologues (Lonhienne et al, 2000), indicates that the high kcat of these at low temperatures is due to a decrease of the activation enthalpy ΔH#—a decrease in the number of enthalpy-driven interactions that have to be broken during catalysis. This decrease is partially compensated by a less favourable activation entropy ΔS#. As a result, and as supported by the negative values of Δ(ΔS#)psychro–meso, the ground-state enzyme–substrate complex shows a broader distribution of conformational states. Consequently, a further effect of the enhanced flexibility should be a looser binding of the substrate, which is observed through high KM values for many psychrophilic enzymes that interact with large substrates (Collins et al, 2002). These catalysts therefore increase kcat at the expense of KM, whereas in some intracellular enzymes this adaptive drift of KM is counteracted by the retention of rigid structural domains (Bentahir et al, 2000).
Another effect of low temperatures on proteins is cold denaturation, a phenomenon that is thought to occur from destabilizing hydration (Makhatadze & Privalov, 1995).
Genomics and proteomics
Three complete genomes have been sequenced so far: those from Desulfotalea psychrophila (Rabus et al, 2004), Colwellia psychrerythraea 34H (Methe et al, 2005) and Pseudoalteromonas haloplanktis TAC125 (Medigue et al, 2005). Draft genome sequences have been produced from two cold-adapted Archaea: Methanogenium frigidum and Methanococcoides burtonii (Saunders et al, 2003). As expected, several Csps and proteins involved in unsaturated fatty-acid synthesis have been identified in these genomes. In addition to the classical lipid desaturases, two gene clusters possibly involved in membrane rigidity/fluidity through the degradation of steroids or hopanoids have been found in the P. haloplanktis genome. Furthermore, β-keto-acyl carrier proteins, β-keto-acyl-CoA synthetases and a fatty-acid cis–trans isomerase have been identified in C. psychrerythraea and could enhance membrane fluidity depending on either their cold-adapted activity or their upregulated expression.
At low temperatures, the solubility of gasses and the production of toxic reactive oxygen species (ROS) increase significantly. To counteract this, C. psychrerythraea and D. psychrophila have an enhanced antioxidant capacity owing to the presence of several genes that encode catalases and superoxide dismutases. By contrast, P. haloplanktis has evolved by suppressing a series of activities that give rise to ROS—for example, the entire molybdopterin metabolism pathway is absent.
The amino-acid composition of the proteome has also been discussed in terms of temperature adaptation. Saunders and co-workers (2003) showed that working from psychrophilic to thermophilic Archaea, there is a trend in increasing leucine content and decreasing glutamine and threonine content. However, this observation was not corroborated by work on D. psychrophila, and an N-driven bias (increase in asparagine content) in the proteins from P. haloplanktis has been shown. Therefore, attempts to find specific adaptations to cold by comparing the proteins from various psychrophilic, mesophilic and thermophilic organisms have produced ambiguous results. However, a study published by Khachane and colleagues (2005) found a significant inverse correlation between the uracil content of 16S rRNA and the optimum growth temperature Topt of cultured organisms. They also proposed an algorithm to predict the Topt values of uncultured prokaryotes that might be useful to identify the appropriate cultivation conditions.
The proteomic approach has been used to acquire a global view of cold adaptation at the protein level. Both Csps and Caps are expressed by the psychrophile Arthrobacter globiformis (Berger et al, 1996) and the psychrotroph Aeromonas hydrophila (Imbert & Gancel, 2004), whereas Seo and colleagues (2004) identified more than 30 overexpressed proteins in Bacillus psychrosaccharolyticus under psychrophilic growth conditions. Furthermore, proteins involved in energy metabolism, transcription and translation processes and in protein quality control were identified at 4 °C in Methanococcoides burtonii (Goodchild et al, 2004a) and a prolyl cis–trans isomerase was identified in Shewanella sp. strain SIB1 (Suzuki et al, 2004).
Conclusion
Psychrophilic microorganisms have successfully confronted the two main physical challenges to which they are exposed: low thermal energy and high viscosity, both of which slow metabolic flux. Proteins are the main targets of these adaptations as they control the equilibrium between substrates and products, influx of nutrients, outflow of waste products, macromolecular assemblies, nucleic-acid dynamics and appropriate folding. Their adaptation seems to rely on a higher flexibility of key parts of the molecular structure or of the whole edifice through a decreased stability that partly compensates the freezing effect of low temperatures on the three-dimensional structure. As shown by genomics and proteomics, cold-shock proteins are also highly expressed and can have crucial roles in protein folding, control of nucleic-acid secondary structure, and transcription and translation. Approaches such as genome sequencing will undoubtedly shed new light on other characteristics of these fascinating organisms.

J.-C. Marx, G. Feller, T. Collins, C. Gerday & S. D'Amico
Acknowledgments
We acknowledge the generous support of the Institut Polaire Français, the Fonds National de la Recherche Scientifique (FNRS; Belgium), the Region Wallonne (Belgium) and the European Union. S.D.'A. is a FNRS postdoctoral researcher.
References
- Arthur H, Watson K (1976) Thermal adaptation in yeast: growth temperatures, membrane lipid, and cytochrome composition of psychrophilic, mesophilic, and thermophilic yeasts. J Bacteriol 128: 56–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentahir M, Feller G, Aittaleb M, Lamotte-Brasseur J, Himri T, Chessa JP, Gerday C (2000) Structural, kinetic, and calorimetric characterization of the cold-active phosphoglycerate kinase from the antarctic Pseudomonas sp. TACII18. J Biol Chem 275: 11147–11153 [DOI] [PubMed] [Google Scholar]
- Berger F, Morellet N, Menu F, Potier P (1996) Cold shock and cold acclimation proteins in the psychrotrophic bacterium Arthrobacter globiformis SI55. J Bacteriol 178: 2999–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintalapati S, Kiran MD, Shivaji S (2004) Role of membrane lipid fatty acids in cold adaptation. Cell Mol Biol (Noisy-le-grand) 50: 631–642 [PubMed] [Google Scholar]
- Collins T, Meuwis MA, Stals I, Claeyssens M, Feller G, Gerday C (2002) A novel family 8 xylanase: functional and physico-chemical characterization. J Biol Chem 277: 35133–35139 [DOI] [PubMed] [Google Scholar]
- Collins T, Meuwis MA, Gerday C, Feller G (2003) Activity, stability and flexibility in glycosidases adapted to extreme thermal environments. J Mol Biol 328: 419–428 [DOI] [PubMed] [Google Scholar]
- D'Amico S, Marx JC, Gerday C, Feller G (2003) Activity–stability relationships in extremophilic enzymes. J Biol Chem 278: 7891–7896 [DOI] [PubMed] [Google Scholar]
- Deming JW (2002) Psychrophiles and polar regions. Curr Opin Microbiol 5: 301–309 [DOI] [PubMed] [Google Scholar]
- Feller G, Gerday C (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol 1: 200–208 [DOI] [PubMed] [Google Scholar]
- Fong NJ, Burgess ML, Barrow KD, Glenn DR (2001) Carotenoid accumulation in the psychrotrophic bacterium Arthrobacter agilis in response to thermal and salt stress. Appl Microbiol Biotechnol 56: 750–756 [DOI] [PubMed] [Google Scholar]
- Georlette D, Blaise V, Collins T, D'Amico S, Gratia E, Hoyoux A, Marx JC, Sonan G, Feller G, Gerday C (2004) Some like it cold: biocatalysis at low temperatures. FEMS Microbiol Rev 28: 25–42 [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Hill PJ, Dodd CE, Laybourn-Parry J (2004) Demonstration of antifreeze protein activity in Antarctic lake bacteria. Microbiology 150: 171–180 [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Davies PL, Laybourn-Parry J (2005) A hyperactive, Ca2+-dependent antifreeze protein in an Antarctic bacterium. FEMS Microbiol Lett 245: 67–72 [DOI] [PubMed] [Google Scholar]
- Goodchild A, Raftery M, Saunders NF, Guilhaus M, Cavicchioli R (2004a) Biology of the cold adapted archaeon, Methanococcoides burtonii determined by proteomics using liquid chromatography-tandem mass spectrometry. J Proteome Res 3: 1164–1176 [DOI] [PubMed] [Google Scholar]
- Goodchild A, Saunders NF, Ertan H, Raftery M, Guilhaus M, Curmi PM, Cavicchioli R (2004b) A proteomic determination of cold adaptation in the Antarctic archaeon, Methanococcoides burtonii. Mol Microbiol 53: 309–321 [DOI] [PubMed] [Google Scholar]
- Imbert M, Gancel F (2004) Effect of different temperature downshifts on protein synthesis by Aeromonas hydrophila. Curr Microbiol 49: 79–83 [DOI] [PubMed] [Google Scholar]
- Inouye M, Phadtare S (2004) Cold shock response and adaptation at near-freezing temperature in microorganisms. Sci STKE 15 June p 26. [DOI] [PubMed] [Google Scholar]
- Jia Z, Davies PL (2002) Antifreeze proteins: an unusual receptor–ligand interaction. Trends Biochem Sci 27: 101–106 [DOI] [PubMed] [Google Scholar]
- Johns GC, Somero GN (2004) Evolutionary convergence in adaptation of proteins to temperature: A4-lactate dehydrogenases of Pacific damselfishes (Chromis spp.). Mol Biol Evol 21: 314–320 [DOI] [PubMed] [Google Scholar]
- Khachane AN, Timmis KN, dos Santos VA (2005) Uracil content of 16S rRNA of thermophilic and psychrophilic prokaryotes correlates inversely with their optimal growth temperatures. Nucleic Acids Res 33: 4016–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krembs C, Eicken H, Junge K, Deming JW (2002) High concentrations of exopolymeric substances in Arctic winter sea ice: implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep-Sea Res I 49: 2163–2181 [Google Scholar]
- Lim J, Thomas T, Cavicchioli R (2000) Low temperature regulated DEAD-box RNA helicase from the Antarctic archaeon, Methanococcoides burtonii. J Mol Biol 297: 553–567 [DOI] [PubMed] [Google Scholar]
- Lonhienne T, Gerday C, Feller G (2000) Psychrophilic enzymes: revisiting the thermodynamic parameters of activation may explain local flexibility. Biochim Biophys Acta 1543: 1–10 [DOI] [PubMed] [Google Scholar]
- Makhatadze GI, Privalov PL (1995) Energetics of protein structure. Adv Protein Chem 47: 307–425 [DOI] [PubMed] [Google Scholar]
- Mancuso Nichols CA, Guezennec J, Bowman JP (2005) Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: a review. Mar Biotechnol 7: 253–271 [DOI] [PubMed] [Google Scholar]
- Medigue C et al. (2005) Coping with cold: the genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res 15: 1325–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methe BA et al. (2005) The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc Natl Acad Sci USA 102: 10913–10918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita RY (1975) Psychrophilic bacteria. Bacteriol Rev 39: 144–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muryoi N, Sato M, Kaneko S, Kawahara H, Obata H, Yaish MW, Griffith M, Glick BR (2004) Cloning and expression of afpA, a gene encoding an antifreeze protein from the arctic plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. J Bacteriol 186: 5661–5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CM, Lardiere SG, Bowman JP, Nichols PD, Gibson JAE, Guezennec J (2005) Chemical characterization of exopolysaccharides from Antarctic marine bacteria. Microb Ecol 49: 578–589 [DOI] [PubMed] [Google Scholar]
- Pandey KD, Shukla SP, Shukla PN, Giri DD, Singh JS, Singh P, Kashyap AK (2004) Cyanobacteria in Antarctica: ecology, physiology and cold adaptation. Cell Mol Biol (Noisy-le-grand) 50: 575–584 [PubMed] [Google Scholar]
- Phadtare S (2004) Recent developments in bacterial cold-shock response. Curr Issues Mol Biol 6: 125–136 [PubMed] [Google Scholar]
- Rabus R et al. (2004) The genome of Desulfotalea psychrophila, a sulfate-reducing bacterium from permanently cold Arctic sediments. Environ Microbiol 6: 887–902 [DOI] [PubMed] [Google Scholar]
- Ratkowsky DA, Olley J, Ross T (2005) Unifying temperature effects on the growth rate of bacteria and the stability of globular proteins. J Theor Biol 233: 351–362 [DOI] [PubMed] [Google Scholar]
- Ray MK, Kumar GS, Shivaji S (1994) Phosphorylation of membrane proteins in response to temperature in an Antarctic Pseudomonas syringae. Microbiology 140: 3217–3223 [DOI] [PubMed] [Google Scholar]
- Russell NJ (1997) Psychrophilic bacteria—molecular adaptations of membrane lipids. Comp Biochem Physiol Physiol 118: 489–493 [DOI] [PubMed] [Google Scholar]
- Russell NJ (2000) Toward a molecular understanding of cold activity of enzymes from psychrophiles. Extremophiles 4: 83–90 [DOI] [PubMed] [Google Scholar]
- Saunders NF et al. (2003) Mechanisms of thermal adaptation revealed from the genomes of the Antarctic Archaea Methanogenium frigidum and Methanococcoides burtonii. Genome Res 13: 1580–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JB, Kim HS, Jung GY, Nam MH, Chung JH, Kim JY, Yoo JS, Kim CW, Kwon O (2004) Psychrophilicity of Bacillus psychrosaccharolyticus: a proteomic study. Proteomics 4: 3654–3659 [DOI] [PubMed] [Google Scholar]
- Siddiqui KS, Bokhari SA, Afzal AJ, Singh S (2004) A novel thermodynamic relationship based on Kramers theory for studying enzyme kinetics under high viscosity. IUBMB Life 56: 403–407 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Haruki M, Takano K, Morikawa M, Kanaya S (2004) Possible involvement of an FKBP family member protein from a psychrotrophic bacterium Shewanella sp. SIB1 in cold adaptation. Eur J Biochem 271: 1372–1381 [DOI] [PubMed] [Google Scholar]
- Tosco A, Birolo L, Madonna S, Lolli G, Sannia G, Marino G (2003) GroEL from the psychrophilic bacterium Pseudoalteromonas haloplanktis TAC 125: molecular characterization and gene cloning. Extremophiles 7: 17–28 [DOI] [PubMed] [Google Scholar]
- Violot S, Aghajari N, Czjzek M, Feller G, Sonan GK, Gouet P, Gerday C, Haser R, Receveur-Brechot V (2005) Structure of a full length psychrophilic cellulase from Pseudoalteromonas haloplanktis revealed by X-ray diffraction and small angle X-ray scattering. J Mol Biol 348: 1211–1224 [DOI] [PubMed] [Google Scholar]
- Vishniac H, Klinger J (1986) Yeasts in the Antarctic deserts. In Megusar F, Gantar M (eds) Perspectives in Microbial Ecology. Proceedings of the 4th ISME p 46–51. Ljubljana, Slovenia: Slovene Society for Microbiology [Google Scholar]
- Wintrode PL, Miyazaki K, Arnold FH (2001) Patterns of adaptation in a laboratory evolved thermophilic enzyme. Biochim Biophys Acta 1549: 1–8 [DOI] [PubMed] [Google Scholar]
- Xu Y, Nogi Y, Kato C, Liang Z, Ruger HJ, De Kegel D, Glansdorff N (2003) Moritella profunda sp. nov. and Moritella abyssi sp. nov., two psychropiezophilic organisms isolated from deep Atlantic sediments. Int J Syst Evol Microbiol 53: 533–538 [DOI] [PubMed] [Google Scholar]
- Yoshimune K, Galkin A, Kulakova L, Yoshimura T, Esaki N (2005) Cold-active DnaK of an Antarctic psychrotroph Shewanella sp. Ac10 supporting the growth of dnaK-null mutant of Escherichia coli at cold temperatures. Extremophiles 9: 145–150 [DOI] [PubMed] [Google Scholar]


