Abstract
Truncation of the tumour suppressor adenomatous polyposis coli (APC) constitutively activates the Wnt/β-catenin signalling pathway. This event constitutes the primary transforming event in sporadic colorectal cancer in humans. Moreover, humans or mice carrying germline truncating mutations in APC develop large numbers of intestinal adenomas. Here, we report that zebrafish that are heterozygous for a truncating APC mutation spontaneously develop intestinal, hepatic and pancreatic neoplasias that are highly proliferative, accumulate β-catenin and express Wnt target genes. Treatment with the chemical carcinogen 7,12-dimethylbenz[a]anthracene accelerates the induction of these lesions. These observations establish apc-mutant zebrafish as a bona fide model for the study of digestive tract cancer.
Keywords: APC, cancer, zebrafish
Introduction
APC (adenomatous polyposis coli) was originally identified as the gene mutated in the familial adenomatous polyposis (FAP) syndrome in humans (Groden et al, 1991; Kinzler et al, 1991). Carriers of germline truncating mutations in APC develop multiple colorectal adenomas following somatic inactivation of the remaining allele (Kinzler & Vogelstein, 1996). Similarly, mice that are heterozygous for a truncating mutation in APC develop multiple tumours in the small intestine (Su et al, 1992). APC is a key inhibitor of the Wnt/β-catenin signalling pathway (Bienz & Clevers, 2000). It is an essential component of the Axin-containing destruction complex that phosphorylates β-catenin, thus tagging it for ubiquitination and degradation by the proteasome. In the presence of a Wnt ligand, β-catenin is stabilized and accumulates in the nucleus, where it associates with T-cell factor (TCF) family transcription factors and activates transcription of target genes. In sporadic cases of colorectal cancer, APC mutations cluster in a region proximal to the central Axin-binding motifs, termed the mutation cluster region (MCR). Truncations at or proximal to the MCR abolish the inhibitory capacity of APC, leading to accumulation of β-catenin and constitutive activity of the Wnt pathway (Korinek et al, 1997). A small percentage of sporadic colorectal cancers without mutated APC turn out to have mutations in the APC-binding domain of β-catenin that has the same effect (Morin et al, 1997).
The zebrafish provides a powerful animal model for genetic analysis of vertebrate embryogenesis, organ development and disease. Zebrafish is unique among vertebrates in its capacity for ‘forward' (phenotype-based) genetic analysis owing to a short generation time, large progeny size and readily accessible transparent embryos. Large-scale forward genetic screens have identified hundreds of mutants with defects in embryogenesis (Driever et al, 1996). Many of these mutants have been proved to be excellent models for a variety of human diseases (Dooley & Zon, 2000). Cancer can occur in the wild in fish (Walter & Kazianis, 2001), and experimental carcinogenesis studies have demonstrated that fish develop tumours in virtually all organs (Spitsbergen et al, 2000). Most importantly, the histopathology of such neoplasia often resembles that of human tumours (Amatruda et al, 2002; Stern & Zon, 2003).
Results
To explore the zebrafish as a model for β-catenin-driven carcinogenesis, we have studied adult heterozygotes carrying a previously identified mutation in the APC gene (Hurlstone et al, 2003). It is noteworthy that homozygous apc mutants show a pleiotropic, embryonic lethal phenotype that included a cardiac valve abnormality (Hurlstone et al, 2003). The truncating mutation (apcMCR) maps in the MCR, and is similar to mutations found in FAP patients. apc/+ fish were killed and assessed by histology for the presence of tumours. As a marker for proliferation, we used proliferating-cell nuclear antigen (PCNA). Elevated levels of cellular β-catenin indicated clonal loss of APC function.
We found that aged apc/+ fish (>15 months, n=34) developed spontaneous tumours, primarily in the liver (6 out of 34) and intestine (4 out of 34), whereas only one wild-type (wt) sibling (n=33) had developed a neoplastic lesion at this age, which located in the liver.
Intestinal adenomas
The organization and self-renewal of the epithelium of adult fish intestine resembles that of neonatal mammals, with proliferation being restricted to the intervillus pockets. Differentiation occurs along the crypt–villus axis (Crosnier et al, 2005; Wallace et al, 2005). With the exception of Paneth cells, fish possess all cell types present in the mammalian intestine. In the wt adult zebrafish intestine, low levels of β-catenin immunoreactivity were detected in the epithelial cells of intestinal villi, largely restricted to the intercellular junctions (Fig 1A). Proliferative PCNA+ cells were restricted to the intervillus pockets (Fig 1D). In 15-month-old apc/+ fish, large outgrowths resembled mammalian polyps. The intestinal architecture was disorganized and in contrast to the ordered periodic arrangement of villi in the wt intestine, large structures with ramifications of the villi, often embedded in fibrovascular stroma, were observed (Fig 1B,C). These lesions were classified as adenomatous polyps, portraying pseudostratification of nuclei, loss of goblet cells and increased nuclear-to-cytoplasmic ratio consistent with dysplastic epithelium. Most of the cells in these intestinal adenomas had accumulated high levels of β-catenin in the cytoplasm and nucleus (Fig 1B,C) and were proliferating as shown by PCNA staining (Fig 1E,F).
Figure 1.
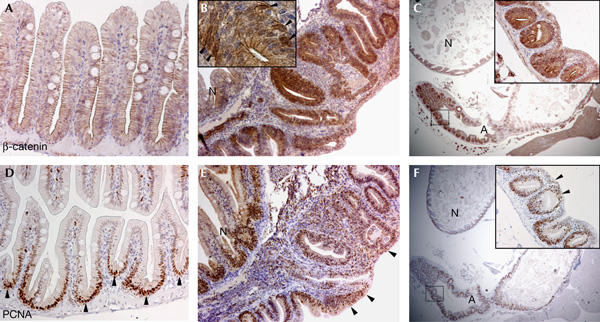
Spontaneous intestinal adenomas in apc/+ fish. (A) Section of wild-type adult fish intestine stained for β-catenin. (B) Section of a large spontaneous polyp from a 15-month-old apc/+ fish stained for β-catenin. Note great disorganization of the intestinal architecture compared with the adjacent normal tissue (N). The inset depicts a high-magnification image of a region of the adenoma in which few cells have accumulated high levels of cytoplasmic and nuclear β-catenin (arrowheads). (C) Section of the intestine from a 20-month-old apc/+ fish stained for β-catenin. A large intestinal adenoma (A) is observed. The inset depicts a high–magnification image of the boxed area of the adenoma. Cells comprising the adenoma express high levels of β-catenin. (D) Immunocytochemistry with proliferating-cell nuclear antigen (PCNA) on adult fish intestine. Proliferation is restricted to the intervillus pockets between the villi (arrowheads). (E) Serial section of the polyp shown in (B) stained for PCNA. Most of the cells in the structure are PCNA+ and ectopic proliferative cells are found in the surface epithelium (arrowheads). (F) Serial section of the adenoma shown in (C) stained for PCNA. Note that almost all the epithelial cells in the neoplasia are proliferating. The inset depicts a higher magnification image of the boxed area of the adenoma in which ectopic proliferation is observed in the surface epithelium (arrowheads). Original magnification: (A,B,D,E) × 200; (C,F) × 20; inset in (B) × 400; inset in (C,F) × 200.
Hepatic adenomas
The adult zebrafish liver abuts the intestine in several locations, instead of being a discrete organ as in mammals. Although unlobulated, the histology of the zebrafish liver is otherwise similar to that of the mammalian liver and consists of differentiated hepatocytes, bile ducts, portal tracts and vessels (Fig 2A). In the wt liver, β-catenin labels the membranes of the hepatocytes (Fig 2A) and there is minimal proliferation, as indicated by labelling for PCNA (Fig 2D). In 6 out of 34 apc/+ fish analysed at this age, we observed hepatic neoplasias, which were often associated with foci of hepatocellular alteration, clusters of hepatocytes with a more eosinophilic and a more vacuolated cytoplasm owing to glycogen accumulation (Fig 2C). In these lesions, we observed changes in cell morphology, such as condensed chromatin, prominent nucleoli and increased occurrence of apoptotic bodies (Fig 2F). These distinct features are reminiscent of hepatoblastoma in human infants. However, as they occurred in aged fish, they did not formally adhere to the classification criteria of hepatoblastomas. The hepatic lesions were circumscribed and maintained more of the original liver architecture and were tentatively classified as hepatic adenomas. The hepatic adenomas showed nuclear staining for β-catenin, the hallmark of activated Wnt signalling (Fig 2B), and a high degree of proliferation (Fig 2E).
Figure 2.
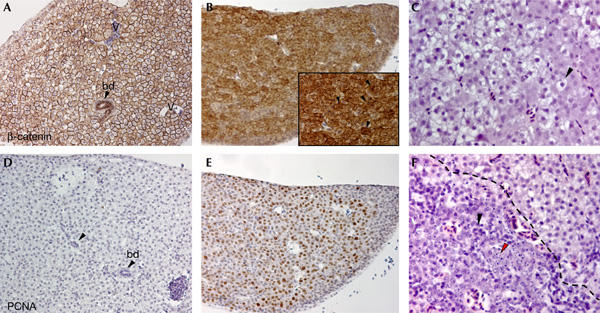
Spontaneous hepatic adenomas in apc/+ fish. (A) Section of a wild-type (wt) zebrafish liver stained for β-catenin. Bile ducts (bd), indicated by arrowheads, and vessels (v) are shown. β-catenin decorates the membranes of hepatocytes. (B) Section depicting a large spontaneous hepatic adenoma encompassing almost an entire liver lobe, from a 15-month-old apc/+ fish stained for β-catenin. Most of the cells in the lesion express high levels of β-catenin. The inset depicts a high-magnification image of some adenoma cells that have accumulated cytoplasmic and nuclear β-catenin (arrowheads). (C) Section of liver from a 14-month-old apc/+ fish stained with haematoxylin–eosin. Cells in this diseased liver appear clear and larger than normal hepatocytes, owing to increased glycogen accumulation (arrowhead). (D) Serial section of the wt liver shown in (A) stained for proliferating-cell nuclear antigen (PCNA). There is almost no proliferation in the adult zebrafish liver. (E) Serial section of the hepatic adenoma shown in (B) stained for PCNA. Most of the cells are proliferating. Note that there is a very good correlation between the PCNA+ cells and β-catenin-expressing cells. (F) In another region of the liver depicted in (C), apoptotic features such as fragmented nuclei (red arrowhead) and prominent nucleoli (black arrowhead) are observed in the lesion (demarcated with a striped line). Original magnification: (A,B,D,E) × 200; inset × 400; (C,F) × 400.
To further characterize the spontaneous intestinal and hepatic lesions in apc/+ fish, we assessed expression of the TCF4 target genes cmyc (He et al, 1998) and axin2 (Jho et al, 2002) by in situ hybridization. In the adult wt fish, cmyc expression is confined to the intervillus pockets of the intestine, and is undetectable in the liver (supplementary Fig 1Aref> online). axin2 expression is barely detectable in the adult fish intestine, and is undetectable in the liver (supplementary Fig 1Bref> online). Most of the cells comprising the intestinal adenomas were expressing high levels of cmyc (Fig 3A) and ectopic axin2 (Fig 3B). In the hepatic adenomas, high levels of cmyc and axin2 expression were found in scattered cells (Fig 3D,E) that were also PCNA positive (Fig 3F). As expected, the intestinal adenomas were negative for the differentiation marker intestinal fatty acid-binding protein (ifabp; Fig 3C). Indeed, ifabp expression is downregulated on activation of Wnt signalling and in adenomas of min mice (van de Wetering et al, 2002). We next assessed the status of the wt APC allele in the lesions. The genomic region encompassing the MCR was amplified and sequenced from adenoma tissue along with adjacent normal tissue (supplementary informationref> online). All samples analysed were heterozygous for apc, indicating that in these adenomas there was no loss of the MCR in the wt allele and also that no other mutations were found in this genomic fragment.
Figure 3.
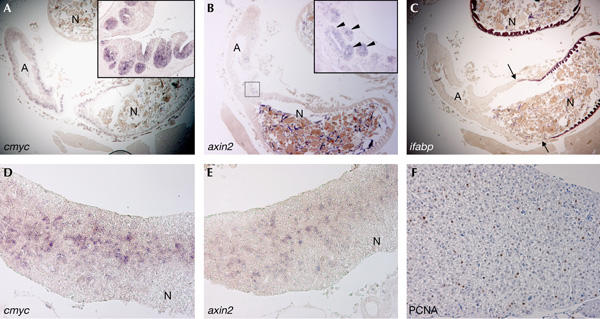
Upregulation of T-cell factor target genes in the adenomas of apc/+ fish. (A) Section of the intestine of a 20-month-old apc/+ fish containing a large intestinal adenoma (A) stained for cmyc. Cells in the adenoma express high levels of cmyc as compared with the normal intestinal tissue (N). The inset depicts a high-magnification image of a region of the adenoma in which most of the epithelial cells in crypt-like structures express high levels of cmyc (purple staining). (B) Serial section of the same intestinal adenoma stained for axin2. The inset depicts a high-magnification image of the boxed area of the adenoma. Epithelial cells in some crypt-like structures express high levels of axin2 (arrowheads). (C) Serial section of the intestinal adenoma stained for intestinal fatty acid-binding protein (ifabp). Note that in contrast to the normal intestinal tissue (N) that is positive for ifabp, the adenoma (demarcated by arrows) is negative for ifabp. (D) Diffuse hepatic neoplasia from a 14-month-old apc/+ fish stained for cmyc. Note that scattered cells in the adenoma are positive for cmyc, whereas those surrounding normal tissue (N) are negative. (E) Serial section of the same hepatic adenoma stained for axin2. Scattered cells in the neoplasia are positive for axin2. (F) Serial section of the same lesion stained for proliferating-cell nuclear antigen. Note scattered proliferative cells (brown staining). Original magnification: (A–C) × 20; (D,E) × 100; (F) × 200; insets in (A,B) × 200.
We then sensitized wt and apc/+ siblings to tumour development by treating them with the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA). DMBA induces point mutations and has been used previously in carcinogenesis experiments in mice and fish (Spitsbergen et al, 2000). Fish from an outcross of apc/+ with wt were treated at 3 weeks of age with 5 p.p.m. DMBA. A total of 82 fish were killed at 6 months of age and a further 58 fish were killed at 14 months of age. We found that DMBA-challenged apc/+ fish developed intestinal, hepatic and pancreatic neoplasms (Table 1). In 35.3% of DMBA-treated apc/+ fish analysed at 6 months, we observed small intestinal adenomas encompassing two or three intervillus pockets. Most of the cells in these lesions including the associated villi and surface epithelium contained high levels of β-catenin and were proliferative (Fig 4A,B). In some cases, we observed structures resembling single overgrown crypts in which cells were piling up in a disorganized fashion, contained high levels of β-catenin and were proliferative (supplementary Fig 2ref> online). These structures resembled dysplastic aberrant crypt foci, premalignant lesions also found in human FAP patients. In the same experiment, 10.4% of wt sibling fish showed similar intestinal lesions. In 44.1% of treated apc/+ fish assessed at 6 months, we observed hepatic neoplasias as compared with one wt fish at that age (2%). The neoplasms contained foci of highly proliferative cells with increased levels of β-catenin (Fig 4C,D). The histology was similar to that of the above-described liver adenomas, which can be considered as premalignant lesions (Spitsbergen et al, 2000). When we analysed older animals (14 months), we observed that a greater percentage of these apc/+ fish showed neoplasms, which were unsurprisingly also larger and more dysplastic (supplementary Fig 2Cref> online). A total of 58.3% of DMBA-treated apc/+ fish showed intestinal neoplasms compared with 20.5% of DMBA-treated wt fish. Liver lesions occurred in 70.8% of apc/+ fish, whereas only 20.5% of wt fish analysed at this age (n=34) showed β-catenin-positive hepatic adenomas. In DMBA-treated livers, we also observed bile duct neoplasias, characterized by abnormal proliferation of the bile ductules and accumulation of β-catenin (supplementary Fig 3ref> online). We observed these bile duct neoplasms in 14.7% of 6-month-old apc/+ fish compared with 4% of wt fish analysed at this age. A total of 29% of 14-month-old apc/+ fish showed bile duct neoplasms, whereas 8.8% of wt fish showed this tumour type. Individual DMBA-treated apc/+ fish typically showed neoplasms in several digestive tract organs, whereas neoplasias were almost always confined to one organ in wt siblings.
Table 1.
Percentages of fish showing Wnt-induced neoplasia in the liver, SI, bile duct and pancreas
| Liver | SI | Pancreas | Bile duct | |
|---|---|---|---|---|
| wt 14 months, n=33 | 3 | 0 | 0 | 0 |
| apc 14 months, n=34 | 17.6 | 11.8 | 0 | 0 |
| wt DMBA 6 months, n=48 | 2.1 | 10.4 | 2.1 | 4.1 |
| apc DBMA 6 months, n=34 | 44.1 | 35.3 | 35.3 | 14.7 |
| wt DMBA 14 months, n=34 | 20.5 | 20.5 | 17.1 | 8.8 |
| apc DMBA 14 months, n=24 | 70.8 | 58.3 | 54.2 | 29.2 |
| DMBA, 7,12-dimethylbenz[a]anthracene; SI, small intestine; wt, wild type. A lesion is scored as a Wnt-induced neoplasia when cells in the tissue have accumulated high levels of β-catenin and are proliferating as assessed by proliferating-cell nuclear antigen staining. | ||||
Figure 4.
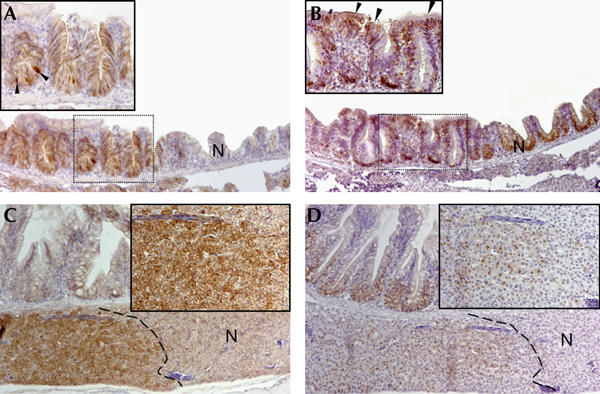
Intestinal and hepatic adenomas in 7,12-dimethylbenz[a]anthracene-treated apc/+ fish. (A) Section of the intestine of a 6-month-old 7,12-dimethylbenz[a]anthracene (DMBA)-treated apc/+ fish stained with β-catenin. A region with high levels of β-catenin compared with the adjacent normal tissue (N) is observed. The inset depicts a high-magnification image of the boxed area of the adenoma in which cells have accumulated high levels of cytoplasmic and nuclear β-catenin (arrowheads). (B) Serial section of the adenoma shown in (A) stained for proliferating-cell nuclear antigen (PCNA). Note that most of the cells in the structure are proliferating. The inset depicts a higher magnification image of the boxed area of the adenoma. Ectopic PCNA+ cells are found in the surface epithelium (arrowheads). (C) Section of liver and small intestine of a 6-month-old DMBA-treated apc/+ fish stained for β-catenin. A confined liver lesion demarcated by a striped line with high levels of β-catenin is depicted. The inset shows a high-magnification image of hepatocytes that have accumulated nuclear and cytoplasmic β-catenin. (D) Serial section of the lesion shown in (C) stained for PCNA. Cells in the lesion (demarcated by a striped line) are proliferating. The inset depicts a higher magnification image of PCNA+ cells. Original magnification: (A–D) × 100; insets × 200.
Pancreatic neoplasias were also observed in DMBA-treated apc/+ fish. In the adult wt pancreas, β-catenin levels were low, and very limited proliferation was observed (Fig 5A,B). In 35.3% of challenged apc/+ fish analysed at 6 months, we observed large masses of acinar cells showing high levels of β-catenin and excessive proliferation (Fig 5C,D). These tumours invariably involved the exocrine pancreas and never endocrine pancreas components. Tumours led to an overall expansion of the organ. Tumour cells showed dysplastic features and had often lost their secretory granules, resembling acinar cell adenomas rather than pancreatic duct lesions. Only 1 out of 48 (2%) DMBA-treated wt fish analysed at 6 months showed a pancreatic tumour.
Figure 5.
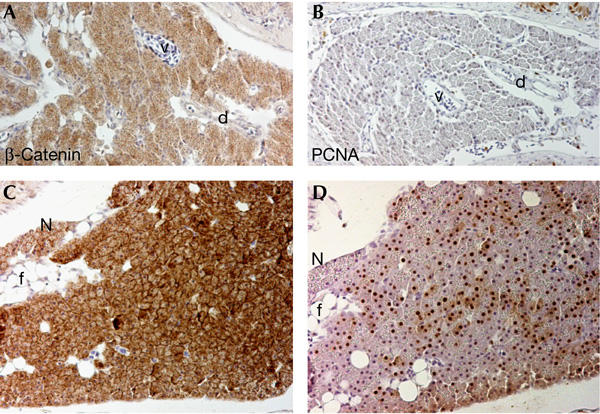
Pancreatic acinar cell adenomas in 7,12-dimethylbenz[a]anthracene-treated apc/+ fish. (A) Section depicting the pancreas of a wild-type zebrafish stained for β-catenin. Note weak diffuse staining in pancreatic acinar cells. d, duct; v, blood vessel. (B) Serial section of the same pancreas stained with proliferating-cell nuclear antigen (PCNA). There is almost no proliferation in the adult pancreas. (C) Section of a pancreatic neoplasia from a 7,12-dimethylbenz[a]anthracene-treated 14-month-old apc/+ fish stained for β-catenin. High levels of cytoplasmic and occasionally nuclear β-catenin are observed in the acinar cells. Note that secretory zymogenic granules seem to be diminished in the acinar cells. A small area of adjacent normal pancreas (N) is presented for comparison. f, fat cells. (D) Serial section of the same lesion stained for PCNA. Most of the cells are proliferating. Original magnification: (A–D) × 200.
Discussion
The current data establish that zebrafish APC, like its mammalian counterpart, is a bona fide tumour suppressor. Moreover, heterozygous loss of APC in the zebrafish appears to phenocopy the cancer phenotype in mammals: (i) the morphology and histopathological features of the neoplasias that we observed resemble those found in humans (this study; Amatruda et al, 2002) and (ii) the spectrum of organs that are affected by neoplastic lesions in APC-mutant fish overlaps with that observed in FAP patients and min mice. In FAP patients, there is a high increase in the likelihood of developing liver and pancreatic tumours in addition to the intestinal tumours (Giardiello et al, 1991, 1993).
We did not detect somatic loss of the wt APC allele in the lesions we analysed, similar to the situation in FAP patients (Nagase & Nakamura, 1993). Nevertheless, our results show that APC function is lost in the cells comprising the adenomas, as documented by the accumulation of β-catenin, the lack of differentiation and the upregulation of Wnt signalling target genes. We assumed that mutations could have occurred elsewhere in the large apc gene, or that the wt APC allele was silenced by epigenetic changes.
The great benefit of modelling cancer in zebrafish is the feasibility of performing genetic or chemical screens for modifiers of the cancer phenotype. This could ultimately lead to the identification of novel therapeutic targets. The early embryonic lethal phenotype of apc homozygous mutants (Hurlstone et al, 2003) could serve as a readout in screens to identify molecules or genes that could suppress or attenuate the cardiac phenotype. Once identified, these genetic or chemical modifiers could then be tested to assess their role in cancer incidence. Current fish models of cancer show unusual tissue tropism as with tp53 and ribosomal protein mutants that develop peripheral nerve sheath tumours (Amsterdam et al, 2004; Berghmans et al, 2005), or use transgenic overexpression of mammalian oncogenes to induce a cancer phenotype, such as activated BRAF in the case of melanoma in fish (Patton et al, 2005) or c-Myc in the case of pancreatic neuroendocrine cancer (Yang et al, 2004) or T-cell leukaemia (Langenau et al, 2003). The apc/+ fish could serve as a valuable model for screens, as the model maintains the sporadic nature of tumours, and closely follows the molecular pathogenesis of its mammalian counterpart.
Methods
Fish. The apcmcr allele has previously been described (Hurlstone et al, 2003). Fish were maintained in the AB or TL genetic backgrounds. Fish were cared for in accordance with institutional guidelines. Fish in the AB background were used for the DMBA studies. In the spontaneous cancer incidence studies, no difference in the incidence of neoplasias between the two backgrounds was observed. Genotyping of fish was carried out by PCR on isolated genomic DNA from tail clips, as described (Hurlstone et al, 2003).
7,12-Dimethylbenz[a]anthracene treatment. wt and apc/+ fry at 3 weeks of age were immersed overnight in a solution containing 5 p.p.m. of DMBA (Sigma-Aldrich-Chemie BV, The Netherlands) in dimethyl sulphoxide. The next day, fry were rinsed several times in water and were returned to the aquarium. Fish were monitored regularly for signs of sickness or evidence of tumours. Fish were killed for analysis at 6 or 14 months after the DMBA treatment.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400638-s1.pdf).
Supplementary Material
Supplementary Information
Acknowledgments
We thank H. Stern (Harvard Medical School, USA) for advice on DMBA treatment and S. Schulte-Merker (Hubrecht Laboratory) for a critical reading of the manuscript. This work was supported by a Dutch Cancer Society (KWF) grant to H.J.C. and in part by an NWO-Vidi grant to A-P.G.H.
References
- Amatruda JF, Shepard JL, Stern HM, Zon LI (2002) Zebrafish as a cancer model system. Cancer Cell 1: 229–231 [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Sadler KC, Lai K, Farrington S, Bronson RT, Lees JA, Hopkins N (2004) Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol 2: 690–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghmans S et al. (2005) tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci USA 102: 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M, Clevers H (2000) Linking colorectal cancer to Wnt signaling. Cell 103: 311–320 [DOI] [PubMed] [Google Scholar]
- Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, Lewis J (2005) Delta–Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 132: 1093–1104 [DOI] [PubMed] [Google Scholar]
- Dooley K, Zon LI (2000) Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev 10: 252–256 [DOI] [PubMed] [Google Scholar]
- Driever W et al. (1996) A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123: 37–46 [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Offerhaus GJ, Krush AJ, Booker SV, Tersmette AC, Mulder JW, Kelley CN, Hamilton SR (1991) Risk of hepatoblastoma in familial adenomatous polyposis. J Pediatr 119: 766–768 [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Offerhaus GJ, Lee DH, Krush AJ, Tersmette AC, Booker SV, Kelley NC, Hamilton SR (1993) Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 34: 1394–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden J et al. (1991) Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66: 589–600 [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512 [DOI] [PubMed] [Google Scholar]
- Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H (2003) The Wnt/β-catenin pathway regulates cardiac valve formation. Nature 425: 633–637 [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F (2002) Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22: 1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87: 159–170 [DOI] [PubMed] [Google Scholar]
- Kinzler KW et al. (1991) Identification of FAP locus genes from chromosome 5q21. Science 253: 661–665 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a β-catenin–Tcf complex in APC−/− colon carcinoma. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- Langenau DM et al. (2003) Myc-induced T cell leukemia in transgenic zebrafish. Science 299: 887–890 [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW (1997) Activation of β-catenin–Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275: 1787–1790 [DOI] [PubMed] [Google Scholar]
- Nagase H, Nakamura Y (1993) Mutations of the APC (adenomatous polyposis coli) gene. Hum Mutat 2: 425–434 [DOI] [PubMed] [Google Scholar]
- Patton EE et al. (2005) BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol 15: 249–254 [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Tsai HW, Reddy A, Miller T, Arbogast D, Hendricks JD, Bailey GS (2000) Neoplasia in zebrafish (Danio rerio) treated with 7,12-dimethylbenz[a]anthracene by two exposure routes at different developmental stages. Toxicol Pathol 28: 705–715 [DOI] [PubMed] [Google Scholar]
- Stern HM, Zon LI (2003) Cancer genetics and drug discovery in the zebrafish. Nat Rev Cancer 3: 533–539 [DOI] [PubMed] [Google Scholar]
- Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF (1992) Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256: 668–670 [DOI] [PubMed] [Google Scholar]
- van de Wetering M et al. (2002) The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250 [DOI] [PubMed] [Google Scholar]
- Wallace KN, Akhter S, Smith EM, Lorent K, Pack M (2005) Intestinal growth and differentiation in zebrafish. Mech Dev 122: 157–173 [DOI] [PubMed] [Google Scholar]
- Walter RB, Kazianis S (2001) Xiphophorus interspecies hybrids as genetic models of induced neoplasia. Ilar J 42: 299–321 [DOI] [PubMed] [Google Scholar]
- Yang HW, Kutok JL, Lee NH, Piao HY, Fletcher CD, Kanki JP, Look AT (2004) Targeted expression of human MYCN selectively causes pancreatic neuroendocrine tumors in transgenic zebrafish. Cancer Res 64: 7256–7262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


