Abstract
The CDK11 (cyclin-dependent kinase 11) gene has an internal ribosome entry site (IRES), allowing the expression of two protein kinases. The longer 110-kDa isoform is expressed at constant levels during the cell cycle and the shorter 58-kDa isoform is expressed only during G2 and M phases. By means of RNA interference (RNAi), we show that the CDK11 gene is required for mitotic spindle formation. CDK11 RNAi leads to mitotic checkpoint activation. Mitotic cells are arrested with short or monopolar spindles. γ-Tubulin as well as Plk1 and Aurora A protein kinase levels are greatly reduced at centrosomes, resulting in microtubule nucleation defects. We show that the mitotic CDK11p58 isoform, but not the CDK11p110 isoform, associates with mitotic centrosomes and rescues the phenotypes resulting from CDK11 RNAi. This work demonstrates for the first time the role of CDK11p58 in centrosome maturation and bipolar spindle morphogenesis.
Keywords: mitosis, polo-like kinase, γ-tubulin, Aurora A
Introduction
The PITSLRE protein kinases belong to a subfamily of p34cdc2-related protein kinases. Ten years ago, PITSLRE protein kinases were renamed CDK11 (cyclin-dependent kinase 11) because of their possible interaction with the regulatory subunits, cyclins L (Xiang et al, 1994). Two proteins, CDK11p110 and CDK11p58, are translated from a single transcript (Cornelis et al, 2000). The CDK11p110 protein kinase is present at constant levels during the cell cycle. The CDK11p110 isoform is involved in pre-RNA splicing and possibly in the regulation of RNA transcription (Trembley et al, 2002). In contrast, the smaller CDK11p58 isoform is cell-cycle regulated, and its synthesis occurs through an internal ribosome entry site (IRES), which is used only during the G2/M transition (Cornelis et al, 2000). Thus, CDK11p58 protein kinase levels become maximal shortly before entry into mitosis.
In this study, we have characterized the behaviour of human tissue culture cells following CDK11 protein knockdown using RNA interference (RNAi). We show that CDK11p58 is a kinase that is, unlike the longer CDK11p110 isoform, active during mitosis when it associates with centrosomes, and has a crucial role in maturation of this organelle and bipolar spindle formation.
Results
CDK11 localizes to nuclear punctae and centrosomes
We began our investigation into CDK11 function by raising a polyclonal antibody that recognizes CDK11p110 and CDK11p58. Protein samples from control (Fig 1A, lanes marked −) or RNAi-treated cells (lanes marked +) were analysed by western blot. Whereas CDK11 proteins were specifically depleted, γ-tubulin levels remained unaffected. These observations confirm the specificity of our CDK11 antibody and indicate that RNAi allows efficient CDK11 ‘knockdown' in HeLa cells.
Figure 1.
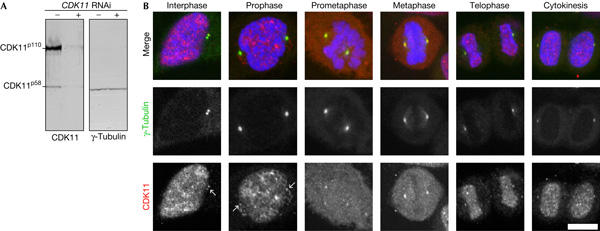
Cyclin-dependent kinase 11 antibody and localization. (A) Western blot showing cyclin-dependent kinase 11 (CDK11; left) protein levels in control (−) and CDK11 RNA interference (RNAi)-treated cells (+). Treatment depletes CDK11 but not the γ-tubulin loading control (right). (B) Immunolocalization of CDK11 protein kinases in HeLa cells throughout the cell cycle. In interphase and early prophase, CDK11 (red and lower panels) is sequestered into nuclei, in which it forms distinct speckles/punctae on the DNA (blue). Colocalization with γ-tubulin (green and middle panels) shows that CDK11 associates with centrosomes throughout the cell cycle. Centrosomal labelling of CDK11 increases as the bipolar spindle forms and the chromosomes congress. This signal diminishes as cells transit into anaphase. During telophase and cytokinesis, CDK11 again becomes concentrated within the reforming nuclei. Scale bar, 10 μm.
We next examined the subcellular localization of CDK11 throughout the cell cycle (Fig 1B). The CDK11 antibody decorated punctae in interphase and early prophase nuclei and was also seen diffusely throughout the cytoplasm (Loyer et al, 1998) and faintly at the centrosomes (arrows). Progression into prophase was marked by an increase in the centrosomal signal. Centrosome staining increased further and seemed to be maximal at prometaphase and metaphase. During telophase and cytokinesis, the kinase re-accumulated in the karyomeres and a low level of centrosome staining remained. Thus, either one or both of the CDK11 protein kinase isoforms preferentially concentrate on mitotic centrosomes.
CDK11 is required for centrosome maturation
The recruitment of CDK11 to mitotic centrosomes raised the possibility that CDK11 might have a role in cell division. To test this hypothesis, we studied CDK11's function using RNAi. Three days after short interfering RNA (siRNA) transfection, CDK11p110 and CDK11p58 protein levels could be reduced by up to 90% (Fig 1A) on western blots, and staining was abolished in fixed cells (not shown). Control and RNAi-treated cells were fixed and stained for microtubules, DNA and centrosomes (Fig 2). The proportion of cells at each of the mitotic stages (Table 1) and the consequences of CDK11 depletion on the spindle were examined (see below).
Figure 2.
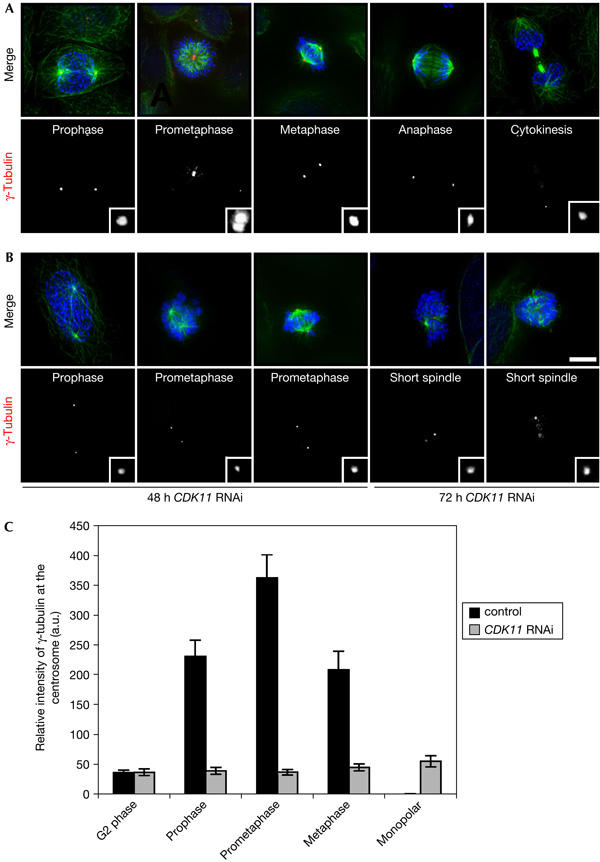
Cyclin-dependent kinase 11 RNA interference leads to monopolar and short spindles with diminished levels of centrosomal γ-tubulin. Distribution of β-tubulin (green), γ-tubulin (red and lower panels in monochrome) and DNA (blue) throughout mitosis in control (A) or CDK11 (cyclin-dependent kinase 11) RNA interference (RNAi)-treated cells (B). During mitosis, γ-tubulin is recruited to the centrosomes and reaches a maximum during prometaphase (insets). (B) The spindles in CDK11 RNAi cells are short and one or more chromosomes often lag at the spindle poles 48 h after treatment. At 72 h after treatment, most chromosomes show congression defects on spindles that are poorly defined or are monopolar. Note the hypercondensed chromosomes in these cells. Scale bar, 10 μm. (C) Relative quantification of γ-tubulin immunofluorescence signal intensity at the centrosomes in control (black) and CDK11 RNAi-treated cells (grey). In controls, centrosomal γ-tubulin levels are maximal during prometaphase, but consistently at least 5–7 times higher in mitosis relative to G2 stages. After depletion of CDK11, the centrosomal γ-tubulin signal intensity does not increase beyond G2 levels.
Table 1.
Quantification of mitotic defects after 48 and 72 h CDK11 RNAi in HeLa cells
| Transfection time | Treatment | Mitotic index* | Prophase* | Prometaphase* | Metaphase* | Anaphase+telophase* | Cytokinesis* | Abnormal metaphase plate*,‡ | Monopolar+short spindles* |
|---|---|---|---|---|---|---|---|---|---|
| 48 h | Control | 8.3±0.5 | 4.4±2.3 | 5.7±1.3 | 16.0±2.2 | 3.5±1.3 | 70.1±2.7 | 0 | 0 |
| CDK11 RNAi | 15.9±0.9 | 3.5±0.6 | 15.6±1.8 | 5.7±2.4 | 1.9±1.0 | 45.8±8.0 | 4.5±2.8 | 22.5±3.4 | |
| 72 h | Control | 9.9±0.3 | 4.3±2.4 | 2.5±1.2 | 24.8±1.4 | 2.8±1.9 | 65.5±3.3 | 0 | 0 |
| CDK11 RNAi | 32.0±1.8 | 1.9±0.7 | 1.1±0.1 | 3.5±0.8 | 0.7±0.6 | 27.9±1.1 | 4.0±0.6 | 60.8±1.8 | |
| CDK11, cyclin-dependent kinase 11; RNAi, RNA interference. | |||||||||
| * Values are a percentage of the total mitosis in each category ±s.d. | |||||||||
| ‡ Metaphase plate with several unaligned chromosomes. | |||||||||
At 48 h after CDK11 RNAi, the number of multinucleated interphase cells increased from 3.7% to 7.9%. A greater increase was observed 72 h after RNAi, and the number of multinucleated cells was elevated by almost five times to that seen in controls. The mitotic index increased by factors of 2 and 3.5 at 48 and 72 h after CDK11 RNAi treatment, respectively. Depleting CDK11 raised the number of abnormal mitoses to about 61% of the total dividing cells examined (Table 1).
At 48 h after CDK11 RNAi, about 23% of the mitotic cells consisted of normal bipolar spindles to which one or more chromosomes seemed to linger at the spindle poles, giving an exaggerated prometaphase-like state (Fig 2B). At 72 h after treatment, in about 61% of the mitotic cells examined, the spindles were abnormally short or monopolar, with hypercondensed chromosomes (Fig 2B). The kinetochores in CDK11 RNAi-treated cells stained positive with the BubR1 checkpoint protein, suggesting improper attachment. Consistent with this, we detected another BubR1-positive band on western blots following CDK11 RNAi, which is a signature of checkpoint activation (Chan et al, 1999; supplementary Fig S1A,B online). These observations indicate that depletion of CDK11 affects the spindle shape, leading to checkpoint activation and mitotic arrest.
We also noticed a reduction in γ-tubulin levels at the centrosomes in CDK11 knockdown cells relative to similarly staged prometaphase control cells (compare Fig 2A and B, insets), and therefore examined the recruitment of γ-tubulin in fixed cells. During prophase in control cells, the γ-tubulin signal at the centrosomes was 4–5 times higher than that in G2 (Fig 2C), and recruitment continued to increase during the first portion of mitosis, such that by late prometaphase the levels were about seven times higher than those seen in G2 (Fig 2C). Although total cellular levels of γ-tubulin were similar between control and CDK11 RNAi cells (Fig 1A), the γ-tubulin signal intensity at the centrosome did not increase beyond that seen in control G2 cells, irrespective of mitotic stage or spindle morphology (Fig 2C). These data strongly suggest that γ-tubulin is not properly recruited to the centrosomes in dividing CDK11-depleted cells.
Microtubule nucleation is facilitated by CDK11
To determine whether the short/monopolar spindles observed after CDK11 RNAi resulted from a failure in centrosome separation or subsequent spindle collapse, we used time-lapse imaging of HeLa cells expressing green fluorescent protein (GFP)-tagged α-tubulin (Fig 3A). In control cells, the time between prometaphase onset and anaphase entry was 90±12 min (n=15; Fig 3A; supplementary Movie 1 online). During this period, the previously separated centrosomes and associated asters formed a bipolar spindle that reached a steady-state metaphase length in about 20 min. The centrosomes were also separated following mitotic entry at prophase in cells 72 h after CDK11 RNAi treatment (Fig 3A, panel 0 min; supplementary Movie 2 online). However, they did not undergo further separation and, in some instances, they seemed to become more closely opposed during the 2 h filming period (n=17), leading to the short spindles observed in our fixed cell studies. Therefore, CDK11 mediates bipolar spindle morphology by promoting centrosome separation.
Figure 3.
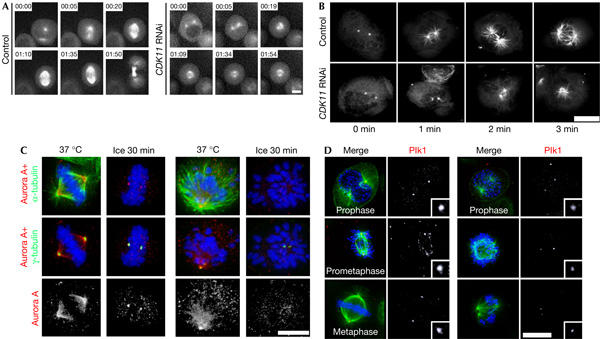
Cyclin-dependent kinase 11 depletion prevents efficient bipolar spindle formation, microtubule nucleation and centrosomal recruitment of Aurora A and Plk1 proteins. (A) Selected frames from time-lapse recordings of mitosis in a control (left) and a CDK11 (cyclin-dependent kinase 11) RNA interference (RNAi)-treated cell (right) expressing green fluorescent protein (GFP)-tagged α-tubulin. In control cells, the centrosomes are already partially separated by prophase (00:00) and maintain this separation or move further apart during spindle formation (00:05). By metaphase (00:20–01:10), the spindle reaches a steady-state length before elongating after anaphase onset (01:35) and cytokinesis (01:50; supplementary Movie 1 online). In this CDK11-depleted cell, the centrosomes were partially separated during prophase (00:00) but did not undergo any further movement, resulting in monopolar spindle and mitotic arrest (01:54; supplementary Movie 2 online). Scale bar, 5 μm. (B) CDK11 RNAi-treated cells have reduced microtubule nucleating potential. A microtubule regrowth assay was performed following cold-induced depolymerization in control (top) and CDK11-depleted cells (bottom) expressing GFP-tagged α-tubulin. In control cells, the centrosomes nucleate robust microtubule asters after being returned to 37°C for 1 and 2 min. Centrosomes in CDK11 RNAi cells fail to nucleate more than a few short microtubules. After 3 min, a period in which control cells start to form a bipolar spindle, only atrophied asters can be detected in CDK11 RNAi cells. Scale bar, 10 μm. (C) Localization of Aurora A in the presence (37°C) or absence of microtubules (ice) in control (left panels) or CDK11-depleted mitoses (right panels). In a control metaphase cell (left), Aurora A (red and lower panel) localizes with the centrosomes (γ-tubulin; green in the middle panels) and spindle microtubules (α-tubulin; green in the top panels). After cold-induced microtubule depolymerization, the kinase still associates with the centrosomes. In the presence of microtubules, CDK11 knockdown (left panels) does not prevent centrosome or spindle microtubule association of Aurora A. In the absence of microtubules, CDK11 RNAi cells fail to accumulate Aurora A kinase at their centrosomes. (D) Efficient recruitment of Plk1 to centrosomes and kinetochores requires CDK11. In control cells, Plk1 (red in the left-hand panels, insets at right are enlargements of the bottom-most centrosome in each corresponding panel) accumulates at the centrosomes and kinetochores as cells progress into metaphase. In CDK11 RNAi cells (right panels), the levels of Plk1 at the centrosomes and kinetochores are markedly reduced (compare control and CDK11 RNAi columns). Scale bar, 10 μm, insets are a × 5 magnification.
As prometaphase centrosome separation is a microtubule-mediated event and CDK11 RNAi hinders centrosomal recruitment of γ-tubulin, we investigated whether the microtubule nucleating potential of this organelle was compromised. We therefore performed microtubule regrowth assays in HeLa cells expressing GFP-tagged α-tubulin (Fig 3B; Meraldi & Nigg, 2001). Following cold-mediated microtubule depolymerization, both control and RNAi-treated cells were returned to 37°C for 1, 2 or 3 min and then fixed for fluorescence analysis. The centrosomes in mitotic control cells nucleated robust microtubule asters in the first minute, and by the third minute nascent spindles could be detected (Fig 3B). By contrast, the centrosomes in CDK11 RNAi cells were difficult to detect and the asters consisted of only a few short microtubules after 1 min (Fig 3B). Prolonging the time did not substantially increase aster density. Thus, in CDK11 knockdown cells, γ-tubulin is not properly recruited to the centrosome and the microtubule nucleation potential of this structure is diminished.
Aurora A and Plk1 recruitment requires CDK11
The phenotype of CDK11 RNAi cells was similar to that observed after Aurora A or Plk1 gene disruptions (Giet et al, 2002; Sumara et al, 2004; Van Vugt et al, 2004). We therefore examined whether the localizations of Aurora A and Plk1 protein kinases were altered after CDK11 RNAi (Fig 3C,D). Western blots (supplementary Fig S2 online) of total cell extracts from asynchronous cultures of control (supplementary Fig S2 online, lane C) and CDK11-depleted cells (supplementary Fig S2 online, lane R) indicated that both Aurora A and Plk1 proteins were increased (supplementary Fig S2A online, middle and lower panels). We believe that this reflects an increase in mitotic cells, a stage in which Aurora A and Plk1 levels are maximal (Nigg, 2001; Barr et al, 2004).
Immunostaining showed that Aurora A localized to spindles of both control (Fig 3C, left panels) and CDK11-depleted cells (Fig 3C, right panels). Therefore, CDK11 is not needed for the recruitment of Aurora A protein kinase to the spindle. The localization of Aurora A on spindle microtubules makes it difficult to assess its centrosome association. Therefore, microtubules were depolymerized by cold treatment before cell fixation and staining (Fig 3C). Aurora A was consistently detected at the centrosomes of control (Fig 3C, lower panel) but not of CDK11 RNAi cells.
We next investigated whether Plk1 distribution was disturbed after CDK11 knockdown (Fig 3D). In control cells, Plk1 (Fig 3D, left panels) associates with the centrosomes from prophase (Fig 3D, upper panels) to metaphase (Fig 3D, lower panels). During prometaphase, we also detected Plk1 on the kinetochores of the congressing chromosomes, although this signal decreased as the chromosomes aligned at the metaphase plate (Fig 3D, compare middle panels with lower panels). Depletion of CDK11 diminished Plk1 staining at the centrosomes as well as the kinetochores. Quantification of Plk1 centrosomal staining intensity showed a 2–3 factor diminution relative to controls in all mitotic stages examined: prophase, prometaphase and metaphase (supplementary Fig S3 online; Fig 3D, compare upper panels and insets). Monopolar spindles, which remain arrested for prolonged periods of time, had similarly reduced centrosomal Plk1 staining (Fig 3D, middle and lower panels). Therefore, CDK11 RNAi limits the amount of Plk1 on the centrosome.
GFP–CDK11p58 rescues CDK11 RNAi mitotic phenotype
Immunostaining studies indicated that a pool of CDK11 localized to mitotic centrosomes. To determine whether CDK11p58, CDK11p110 or both isoforms are associated with these structures, we generated two human cell lines stably expressing GFP–CDK11p58 or GFP–CDK11p110 protein kinases (Fig 4A). To prevent the translation of the p58 isoform in GFP–CDK11p110 cells, the IRES sequence was mutated. We further introduced five silent mutations in the open reading frame of both GFP transgenes to give resistance to the siRNA. The endogenous CDK11p110 protein kinase was detected in HeLa, GFP–CDK11p58 and GFP–CDK11p110 cell samples (Fig 4A). In addition, 90- and 140-kDa proteins corresponding to GFP–CDK11p58 and GFP–CDK11p110, respectively, were shown using anti-CDK11 (Fig 4A, left panel) and anti-GFP antibodies (Fig 4A, middle panel). Immunoprecipitates against the GFP–CDK11p58 and GFP–CDK11p110 proteins during interphase (Fig 4B, lanes I) or mitosis (Fig 4B, lanes M) were subjected to western blotting (Fig 4B, upper panel) and in vitro histone H1 kinase assays (Fig 4B, middle panel). The kinase activity of GFP–CDK11p58 was two times higher during mitosis than during interphase (Fig 4B, lower panel). Conversely, GFP–CDK11p110 was four times more active during interphase than during mitosis.
Figure 4.
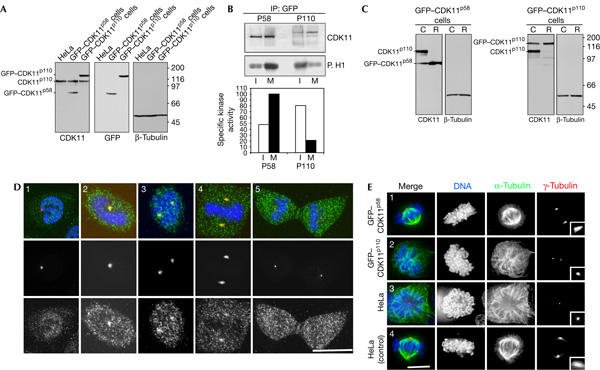
Green fluorescent protein (GFP)–CDK11p58 but not GFP–CDK11p110 is a centrosome-associated active mitotic kinase. (A) Western blots of cell extracts from HeLa cells and HeLa transgenic cell lines expressing GFP–CDK11p58 and GFP–CDK11p110. β-Tubulin is the loading control. (B) Immunoprecipitation (IP) was performed with GFP–CDK11p58 (left) and GFP–CDK11p110 (right) cell extracts during interphase (I) or mitosis (M), and the recovered proteins were subjected to a kinase assay. The upper panel shows a CDK11 western blot and the lower panel, the corresponding histone H1 kinase assay. GFP–CDK11p58 protein kinase is more active during mitosis than interphase, whereas GFP–CDK11p110 is more active during interphase than mitosis. Specific kinase activity determined is shown in the right panel. (C) Western blots of GFP–CDK11p58 and GFP–CDK11p110 cell lines treated with CDK11 short interfering RNA (siRNA). The engineering of silent mutations into each of the GFP–CDK variants makes them resistant to CDK11 RNA interference (RNAi; R), whereas the endogenous proteins are depleted relative to the controls (C). (D) Localization of GFP–CDK11p58. Cells were fixed and stained with an anti-GFP antibody (green and lower panels), anti-pericentrin antibody (red) and co-stained for DNA (blue). GFP–CDK11p58 can be detected in the nucleus and cytoplasm during interphase (1). Following mitotic entry (2), GFP–CDK11p58 is found in the cytoplasm and at the centrosomes. The kinase remains associated with the centrosome during prometaphase (3) and metaphase (4), but is no longer detectable on centrosomes during cytokinesis (5). Scale bar, 10 μm. (E) GFP–CDK11p58 but not GFP–CDK11p110 can rescue the CDK11 RNAi phenotype. The different cell lines were treated with CDK11 RNAi (1–3) and the distribution of tubulin (green), γ-tubulin (red) and DNA (blue) was examined. GFP–CDK11p58 cells depleted of CDK11 (1) were indistinguishable from control cells (4), and formed bipolar spindles. Treatment of GFP–CDK11p110 cells with CDK11 siRNA (2) resulted in the same short or monopolar spindle phenotype seen in HeLa cells (3). Scale bar, 10 μm.
We next studied the distribution of GFP–CDK11p58 and GFP–CDK11p110. In contrast to our immunofluoresence study, GFP–CDK11p58 (Fig 4D) was diffusely nuclear and cytoplasmic during interphase and was not detectable at the centrosomes (Fig 4D, panel 1). This discrepancy may be due to variations in the fixation procedures used in the experiments. Entry into mitosis was marked by the recruitment of the kinase to the centrosomes (Fig 4D, panel 2). GFP–CDK11p58 continued to concentrate on centrosomes during prometaphase and metaphase (Fig 4D, panels 3,4), but by telophase and cytokinesis (Fig 4D, panel 5) this signal was not detectable above the cytoplasmic level seen throughout the cell cycle. By contrast, GFP–CDK11p110 (supplementary Fig S4 online) was never observed on centrosomes and was found in mitotic cytoplasm during karyokinesis. Following nuclear reformation at telophase, the p110 isoform concentrated into nuclei where it remained during interphase. These data suggest that the p58 isoform and not the p110 CDK11 isoform has a mitotic function.
To confirm a mitotic role of the p58 isoform, rescue experiments were performed on the GFP–CDK11 cell lines following CDK11 RNAi. As shown in Fig 4C, the silent mutations engineered into these transgenes conferred resistance to the RNAi, whereas the endogenous proteins were depleted. None of the short or monopolar spindles associated with knockdown of endogenous CDK11 were seen in GFP–CDK11p58-expressing cells (Fig 4E, compare panel 1 with panels 3,4). Further analysis showed that expression of the mutant CDK11p58 transgene completely rescued mitotic progression (Table 2). No rescue was observed in GFP–CDK11p110 cells after CDK11 RNAi, and abnormal spindles and mitotic arrest were prevalent (Fig 4E, panel 2; Table 2). These data indicate that CDK11p58 but not CDK11p110 is a mitotic kinase that localizes to and regulates the maturation of centrosomes for bipolar spindle formation.
Table 2.
Quantification of mitotic defects after 48 h CDK11 RNAi in GFP–CDK11P58 and GFP–CDK11P110 cells
| Cell line | Treatment: | Mitotic index | Prophase* | Prometaphase* | Metaphase* | Anaphase+telophase* | Cytokinesis* | Monopolar+short spindles* |
|---|---|---|---|---|---|---|---|---|
| GFP–CDK1p58 | Control | 10.3±0.6 | 1.5±0.5 | 5.1±1.2 | 13.2±2.1 | 1.5±0.6 | 78.7±3.6 | 0 |
| CDK11 RNAi | 11.3±1.4 | 1.5±0.6 | 4.4±0.8 | 13.8±1.6 | 1.5±0.6 | 78.9±2.7 | 0 | |
| GFP–CDKp110 | Control | 6.9±0.3 | 6.2±2.1 | 13.8±0.9 | 21.1±3.4 | 5.9±1.0 | 53.0±1.2 | 0 |
| CDK11 RNAi | 21.4±3.1 | 3.3±0.7 | 11.4±2.9 | 4.4±0.4 | 4.9±1.9 | 43.5±3.0 | 34.7±2.0 | |
| CDK11, cyclin-dependent kinase 11; RNAi, RNA interference. | ||||||||
| * Values are a percentage of the total mitosis in each category ±s.d. | ||||||||
Discussion
In this paper, we demonstrate for the first time a role for the human CDK11 gene in mitosis. We show that the p58 isoform, but not the p110 isoform, is a centrosome-associated mitotic kinase involved in centrosome maturation and bipolar spindle formation. The regulatory partners of CDK11p58 are at present unknown. We found no evidence for the association of CDK11p58 with the mitotic cyclins A or B or with cyclin L (supplementary Fig S6 online). This latter finding suggests that the kinase activity of p58 is regulated in a manner different from that of the p110 isoform (Xiang et al, 1994).
How does CDK11p58 control spindle bipolarity? Our time-lapse studies indicated that knockdown of CDK11 prevented proper centrosome separation. We cannot rule out a role for CDK11p58 in recruiting or activating the motor proteins involved in this process. However, we observed that CDK11 depletion diminished the centrosomal levels of γ-tubulin, Plk1 and Aurora A, proteins with known roles in microtubule nucleation and spindle stabilization (Giet et al, 2002; Sumara et al, 2004; Van Vugt et al, 2004). It is therefore possible that CDK11 RNAi perturbs centrosome separation not by affecting the motors themselves, but by altering the numbers and/or stability of the microtubule lattices on which they move. Indeed, in the absence of CDK11, centrosomes nucleate far fewer microtubules. Recruitment of the microtubule nucleating protein γ-tubulin to the centrosome requires Plk1 activity, and this family of kinases is in turn regulated by phosphorylation (Qian et al, 1999; Elia et al, 2003). Thus, it is tempting to speculate that Plk1 is a CDK11p58 substrate. It will be of interest to test this possibility and to identify other substrates of this new mitotic kinase and understand the molecular mechanisms by which it controls centrosome composition.
Methods
Cell culture, synchronization and transfection. HeLa cells and HeLa cells stably expressing GFP-tagged α-tubulin, GFP–CDK11p58 or GFP–CDK11p110 were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Cells were synchronized by thymidine double block. Mitotic cells were collected by mitotic shake off after incubating in 1 μg/ml nocodazole for 16 h. Transfection of plasmids was performed using calcium phosphate.
Microtubule regrowth assays. GFP-tagged α-tubulin-expressing cells were transfected with CDK11 or control siRNA oligonucleotides. After 72 h, the microtubules were depolymerized by placing the cells on ice for 30 min. These cells were then incubated with warm (37°C) medium for 1, 2 or 3 min and fixed with −20°C methanol and processed for immunofluorescence microscopy (see the supplementary information online).
Oligonucleotides, siRNAs, antibody production and manipulation. See the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400639-s1.pdf, http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400639-s2.pdf, http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400639-s3.pdf, http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400639-s4.pdf, http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400639-s5.pdf, http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400639-s6.avi, http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400639-s7.avi, http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400639-s8.doc).
Supplementary Material
Supplementary Fig S1
Supplementary Fig S2
Supplementary Fig S3
Supplementary Fig S4
Supplementary Fig S5
Supplementary Movie 1
Supplementary Movie 2
Supplementary Information
Acknowledgments
We thank Dr Kidd, Dr Loyer and Dr Baffet for antibodies, Dr Lahti for communicating results before publication, Dr Cazalès for GFP-tagged α-tubulin cells and Cornelis for CDK11 constructs. We also thank the lab members for critical readings and Dr B. Martin for the maintenance of cell lines. The microscopy work was supervised by Dr Dutertre (IFR 140 GFAS). The ‘Ligue Nationale Contre le Cancer' supported the work. C. Petretti is a fellow of the Region Bretagne.
References
- Barr FA, Sillje HH, Nigg EA (2004) Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol 5: 429–440 [DOI] [PubMed] [Google Scholar]
- Chan GK, Jablonski SA, Sudakin V, Hittle JC, Yen TJ (1999) Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J Cell Biol 146: 941–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis S, Bruynooghe Y, Denecker G, Van Huffel S, Tinton S, Beyaert R (2000) Identification and characterization of a novel cell cycle-regulated internal ribosome entry site. Mol Cell 5: 597–605 [DOI] [PubMed] [Google Scholar]
- Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB (2003) The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell 115: 83–95 [DOI] [PubMed] [Google Scholar]
- Giet R, McLean D, Descamps S, Lee MJ, Raff JW, Prigent C, Glover DM (2002) Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J Cell Biol 156: 437–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyer P, Trembley JH, Lahti JM, Kidd VJ (1998) The RNP protein, RNPS1, associates with specific isoforms of the p34cdc2-related PITSLRE protein kinase in vivo. J Cell Sci 111(Part 11): 1495–1506 [DOI] [PubMed] [Google Scholar]
- Meraldi P, Nigg EA (2001) Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. J Cell Sci 114: 3749–3757 [DOI] [PubMed] [Google Scholar]
- Nigg EA (2001) Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol 2: 21–32 [DOI] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Maller JL (1999) Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol Cell Biol 19: 8625–8632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumara I, Gimenez-Abian JF, Gerlich D, Hirota T, Kraft C, De La Torre C, Ellenberg J, Peters JM (2004) Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr Biol 14: 1712–1722 [DOI] [PubMed] [Google Scholar]
- Trembley JH, Hu D, Hsu LC, Yeung CY, Slaughter C, Lahti JM, Kidd VJ (2002) PITSLRE p110 protein kinases associate with transcription complexes and affect their activity. J Biol Chem 277: 2589–2596 [DOI] [PubMed] [Google Scholar]
- van Vugt MA, van de Weerdt BC, Vader G, Janssen H, Calafat J, Klompmaker R, Wolthuis RM, Medema RH (2004) Polo-like kinase 1 is required for bipolar spindle formation but is dispensable for anaphase promoting complex/Cdc20 activation and initiation of cytokinesis. J Biol Chem 35: 36841–36854 [DOI] [PubMed] [Google Scholar]
- Xiang J, Lahti JM, Grenet J, Easton J, Kidd VJ (1994) Molecular cloning and expression of alternatively spliced PITSLRE protein kinase isoforms. J Biol Chem 269: 15786–15794 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig S1
Supplementary Fig S2
Supplementary Fig S3
Supplementary Fig S4
Supplementary Fig S5
Supplementary Movie 1
Supplementary Movie 2
Supplementary Information


