Abstract
We identified Caspase-8 as a new substrate for Src kinase. Phosphorylation occurs on Tyr380, situated in the linker region between the large and the small subunits of human Procaspase-8, and results in downregulation of Caspase-8 proapoptotic function. Src activation triggers Caspase-8 phosphorylation on Tyr380 and impairs Fas-induced apoptosis. Accordingly, Src failed to protect Caspase-8-defective human cells in which a Caspase-8-Y380F mutant is expressed from Fas-induced cell death. Remarkably, Src activation upon EGF-receptor stimulation triggers endogenous Caspase-8 phosphorylation and prevents Fas-induced apoptosis. Tyr380 is phosphorylated also in human colon cancers where Src is aberrantly activated. These data provide the first evidence for a direct role of tyrosine phosphorylation in the control of caspases and reveal a new mechanism through which tyrosine kinases inhibit apoptosis and participate in tumor progression.
Keywords: apoptosis, cancer, Caspase-8, Fas-receptor, Src non-receptor tyrosine kinase
Introduction
Caspases are key players in apoptosis, yet the mechanisms that regulate their activity and function are still largely obscure. We are investigating the crosstalk between non-receptor tyrosine kinases, central transducers of the proliferative response, and caspases and whether these two families of proteins can modulate each other's function through their enzymatic activities. We have previously reported that caspases can directly cleave c-Abl non-receptor tyrosine kinase modulating its cellular localization and its function (Barilà et al, 2003). Conversely, the overexpression of some kinases protects cells from apoptosis (Hunter, 2000). Recent studies have shown that, during cellular proliferation, Akt and MAPK switch-off the activities of Caspase-9 (Cardone et al, 1998; Allan et al, 2003), Caspase-8 and Caspase-3 (Alvarado-Kristensson et al, 2004) by direct Ser/Thr phosphorylation. However, the role of tyrosine phosphorylation in the control of caspase activity remains almost totally underinvestigated.
The Src family of non-receptor tyrosine kinases has been reported to modulate Fas-signaling. Interestingly, defective expression of the SHP-1 tyrosine phosphatase in lymphoid cells blocks Fas-mediated apoptosis (Su et al, 1995), while in neutrophils SHP-1 can bind death receptors and block cytokine-induced antiapoptotic signaling (Daigle et al, 2002) enforcing the idea that tyrosine dephosphorylation of specific substrates is a prerequisite for death receptor-induced apoptosis.
Fas (CD95/APO-1) is a transmembrane protein belonging to the tumor necrosis factor superfamily. Upon binding of Fas ligand or agonistic antibodies, the Fas receptor recruits several cytosolic proteins to form the death-inducing signaling complex (DISC). This is necessary to catalyze dimerization, activation (Boatright et al, 2003; Chang et al, 2003; Donepudi et al, 2003) and processing (Muzio et al, 1996; Medema et al, 1997) of Procaspase-8 to form the Caspase-8 tetramer, composed of two p18 and two p10 subunits (Blanchard et al, 1999; Watt et al, 1999), and initiate the caspase cascade. Procaspase-8 activation is absolutely required to trigger this apoptotic response (Juo et al, 1998) and its catalytic activity has to be tightly regulated to avoid inappropriate activation and undesired cell death (Peter, 2004).
To investigate the role of Src family kinases in the control of caspases, we tested whether non-receptor tyrosine kinases impinge on Fas-signaling directly targeting Caspase-8. We identified Caspase-8 as a novel substrate of Src tyrosine kinase. Src directly phosphorylates Procaspase-8 on Tyr380 and prevents its activation, and therefore its proapoptotic function upon Fas-receptor stimulation. The Src family of non-receptor tyrosine kinases plays an important role in the transduction of the proliferative signal triggered by growth factors like PDGF, EGF and HGF (Bromann et al, 2004). More importantly, Src kinase activity is upregulated in several epithelial and nonepithelial cancers (Frame, 2002). Here, we show that endogenous Caspase-8 is tyrosine phosphorylated upon EGF receptor stimulation. Moreover, EGF impairs Fas-induced apoptosis, most likely through Caspase-8 phosphorylation, pointing to Caspase-8 tyrosine phosphorylation as a novel mechanism to suppress apoptosis. Remarkably, Caspase-8 is phosphorylated on Tyr380 also when Src kinase activity is pathologically upregulated, such as in colon cancer, highlighting Tyr380 phosphorylation as a potential molecular strategy of apoptosis suppression adopted by tumor cells.
Results
Src kinase triggers Caspase-8 tyrosine phosphorylation
To test whether Caspase-8 could be a substrate for non-receptor tyrosine kinases, we transiently transfected Caspase-8 in human embryo kidney (HEK) 293 cells, together with several constructs encoding different forms of Src and of Abl kinases. Since overexpression of Caspase-8 in mammalian cells is sufficient to trigger its own activation and cell death, all transfections were performed in the presence of the strong caspase inhibitor CrmA (Tewari and Dixit, 1995; Zhou et al, 1997). The constitutively active Src, SrcY527F, but not its kinase inactive counterpart, caused a strong tyrosine phosphorylation of cotransfected Caspase-8, as shown by immunoblotting (Figure 1, right panel) and in immunoprecipitation experiments (Figure 1, left panel). Interestingly, neither the constitutively active mutant of c-Abl, Abl-PP, nor the leukemiogenic form of Abl, Bcr-Abl, could phosphorylate Caspase-8, suggesting that Caspase-8 is specifically phosphorylated by Src.
Figure 1.

Src kinase phosphorylates Caspase-8. HEK293 cells were transiently transfected with Caspase-8, and either Src or Abl kinases. All transfections included pEGFP-N1-CrmA to prevent the induction of apoptosis due to Caspase-8 overexpression. Total protein extracts or immunoprecipitated Caspase-8 were separated by SDS–PAGE and immunoblotted with specific antibodies. The * points to a portion of Caspase-8 that corresponds to the protein translated from the Met43 (Medema et al, 1997). On total immunoblots Src and Caspase-8 migrate at the same Mw, as shown by the arrow. c-Jun has been used as a control substrate of Abl.
Tyrosine phosphorylation protects cells from Fas-induced apoptosis
Caspase-8 is absolutely required for Fas-induced cell death (Juo et al, 1998) and defective Caspase-8 activity results in the impairment of this apoptotic response. To test whether tyrosine phosphorylation could affect Caspase-8 activity and function, HeLa cells, which are sensitive to Fas, were transfected with a construct encoding the constitutively active form of Src, SrcY527F, along with a plasmid encoding GFP to allow detection of transfected cells. SrcY527F overexpression drives tyrosine phosphorylation of endogenous Caspase-8 (Figure 2A). Interestingly, SrcY527F but not its kinase inactive counterpart, SrcY527F-kin−, decreases Fas-induced apoptosis, similarly to the strong caspase viral inhibitor CrmA, indicating that Src activity is necessary to mediate its antiapoptotic effect (Figure 2B).
Figure 2.
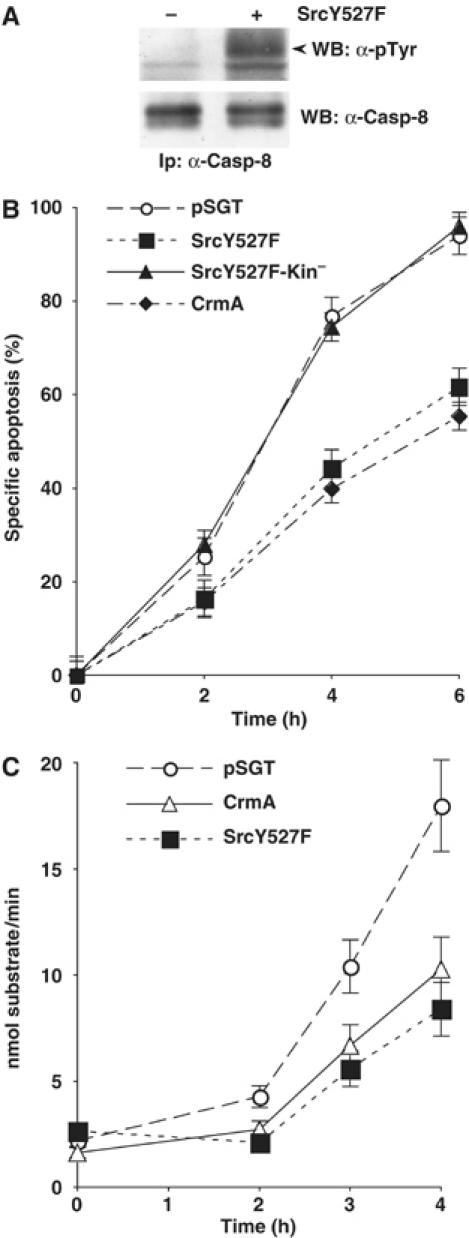
Src kinase activity modulates Fas-induced apoptosis and Caspase-8 activity. (A) HeLa cells were transiently transfected with SrcY527F or with the empty vector as control. Immunoprecipitated Caspase-8 was separated by SDS–PAGE gel and analyzed by immunoblotting with anti-phosphotyrosine and with anti-Caspase-8 antibodies. The arrow indicates Caspase-8. (B) At 24 h after transfection, HeLa cells, transiently transfected with the indicated constructs, were treated with 250 ng/ml anti-Fas and 1 μg/ml cycloheximide. Apoptosis was determined by counting Hoechst-stained fragmented nuclei in GFP-positive cells. The data are presented as the mean value±s.d. of five independent experiments. (C) HeLa cells were transfected with the indicated expression constructs. Apoptosis was induced as in Figure 2B and Caspase-8 activity was analyzed in protein extract by measuring the hydrolysis of the Caspase-8 substrate Ac-IETD-pNA. Data are presented as the mean value±s.d. of three independent experiments.
To further explore whether Src-induced resistance to Fas could be directly linked to Caspase-8 phosphorylation, we analyzed the activation of Caspase-8, upon Fas-stimulation, in HeLa cells transfected with the constitutively active Src. SrcY527F overexpression delayed Fas-induced Caspase-8 activation, measured as its ability to cleave its substrate peptide IETD, to an extent remarkably similar to the strong caspase viral inhibitor CrmA (Figure 2C).
These data suggest that tyrosine phosphorylation affects Caspase-8 proapoptotic function.
Src phosphorylates Caspase-8 on Tyr380
To identify Src-induced tyrosine phosphorylated site(s) on Caspase-8, a Myc-tagged version of Caspase-8 was expressed in HEK293 cells, cotransfected with SrcY527F, and immunoprecipitated. The band corresponding to immunoprecipitated Caspase-8 was visualized by Coomassie staining, recovered from SDS–PAGE gel, and digested with trypsin. The peptide mixture was then treated or not treated with alkaline phosphatase and the phosphorylation profile analyzed by MALDI-TOF mass spectrometry. One phosphopeptide was isolated, corresponding to amino acids 368–391, containing a single phosphate group. The identity of the phosphopeptide was further confirmed by the presence of a methionine residue which, when oxidated, caused a further mass shift of 16 Da. The only tyrosine residue within this peptide is the Tyr380 of human Caspase-8 (Figure 3A). To further investigate whether Tyr380 was the major Src phosphorylation site in Caspase-8, we tested a mutant where Tyr380 was mutated to Phe (Caspase-8-Y380F) in cotransfection assays with SrcY527F or with SrcΔSH3, another constitutively active form of Src (data not shown). Mutation of Tyr380 virtually abrogated tyrosine phosphorylation of Caspase-8, supporting the notion that Tyr380 is the major Src phosphorylation site (Figure 3B).
Figure 3.
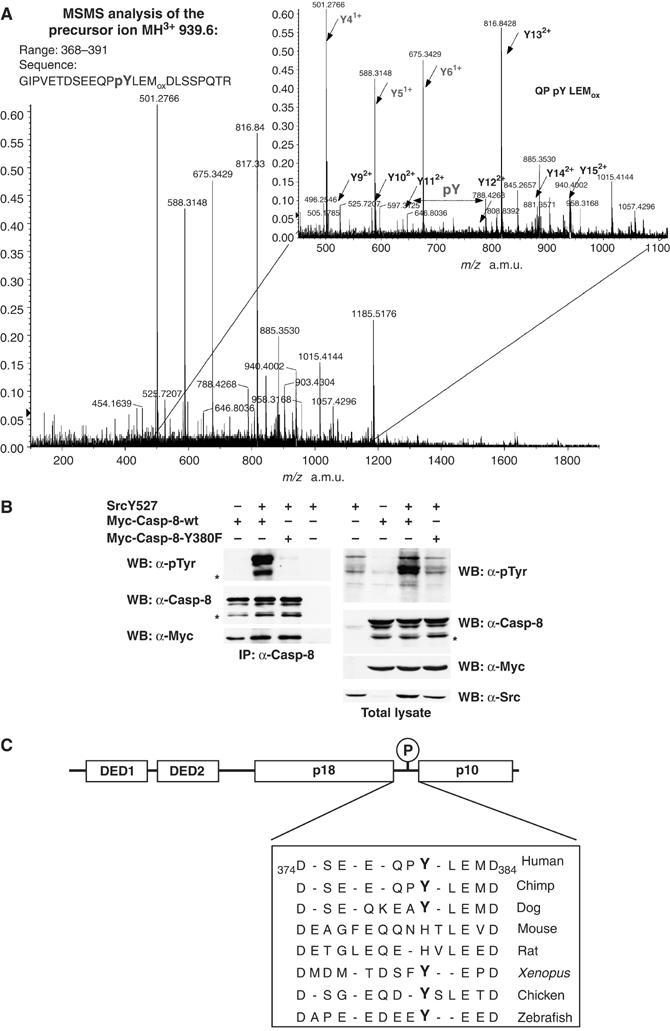
Src kinase phosphorylates Caspase-8 mainly on Tyr380. (A) Myc-Caspase-8 has been immunoprecipitated from extracts prepared from HEK293 cells transiently cotransfected with Myc-Caspase-8, SrcY527F and CrmA. Immunoprecipitated Caspase-8 has been recovered from SDS–PAGE upon Coomassie staining and the protein processed for MS. The picture shows the product ion spectrum of the oxidated form of the phosphopeptide GIPVETDSEEQPpYLEM(ox)DLSSPQTR (precursor ion MH3+=939.6 m/z). The region of the spectrum where the ions corresponding to the sequence QP pY LEM(ox) is enlarged. Peaks corresponding to y′ series are labeled. (B) HEK293 cells were cotransfected with the indicated constructs always along with CrmA. Total protein extracts or immunoprecipitated Caspase-8 were separated by SDS–PAGE and immunoblotted with specific antibodies. (C) Alignment of the putative linker region between the p18 and the p10 subunit of Caspase-8 in different species. Tyrosine residues corresponding toTyr380 on the human sequence have been highlighted in bold.
Interestingly, Tyr380 lies in the linker sequence that separates the large subunit and the small subunit, between the two cleavage residues D374 and D384 (Figure 3C). This region is removed upon activation and it is therefore not part of the structures of the p18–p10 tetrameric complex (Blanchard et al, 1999; Watt et al, 1999). No structural information is currently available on this linker sequence and its function in Caspase-8 regulation remains speculative.
We raised a monoclonal antibody against a phosphopeptide containing phospho-Tyr380. Indeed, this antibody detected Caspase-8 only after Src phosphorylation, whereas mutation of Tyr380 to Phe abolished recognition, demonstrating that the antibody specifically reacts with the phosphorylated site (Supplementary Figure S1).
Src directly phosphorylates Caspase-8 on Tyr 380 and this event impairs Caspase-8 activity in yeast
The yeast Schizosaccharomyces pombe does not express endogenous tyrosine kinases and caspases (Superti-Furga et al, 1993; Silke et al, 2001), and can therefore be used as an in vitro system to address the question whether exogenous Src directly phosphorylates exogenous Caspase-8. Owing to the lack of the physiological regulator Csk, Src is constitutively active in yeast and its activity triggers cell death (Superti-Furga et al, 1993). We observed that Caspase-8 is also highly toxic, depending on its enzymatic activity (data not shown). To allow the expression of high levels of proteins, we coexpressed Src and Caspase-8 under the control of inducible promoters, as previously reported (Superti-Furga et al, 1993). Caspase-8 accumulation in yeast drives its autoprocessing, leading to the ordered production of the p10 (small) and p43 (DEDs-p18) subunits first and then to the release of the p18, detectable by immunoblots with specific antibodies, similarly to what described in the mammalian cells (Figure 4A). Caspase-8 was immunoprecipitated and processed for mass-spectrometry, as described in Figure 3A. Again, Tyr380 was identified as the major phosphorylated residue (data not shown), indicating that Src kinase can directly phosphorylate Caspase-8. The absence of endogenous tyrosine kinases and caspases in S. pombe provides a perfect naïve environment in which to test the effect and the crosstalk of these heterologous proteins (Superti-Furga et al, 1993) on Caspase-8 activity and function. Extracts from S. pombe at different times of protein expression induction, transfected with Src or with the empty vector control along with Caspase-8, were processed for immunoblot analysis using an anti-Caspase-8 antibody raised against the p18 subunit. This antibody detects both the Procaspase-8 and its cleaved p18 subunit. Caspase-8 expression is controlled by an inducible promoter, which allows the detection of Caspase-8 protein by immunoblotting around 10–11 h of induction. At this time we can detect only the presence of Procaspase-8, which further accumulates and is processed and activated at 12, 13 and 14 h. Interestingly, the amount of Procaspase-8 as well as of its cleavage products dramatically decrease at 16 h of induction due to its high toxicity and to consequent cell death (Supplementary Figure S2A). The expression of Src triggers Caspase-8 phosphorylation (data not shown) and results in the delayed accumulation of the p18 subunit at 13 h of induction, supporting the idea that Src kinase activity interferes with Caspase-8 processing and activation (Figure 4B). To further confirm this hypothesis, we analyzed Caspase-8 activation in the same extracts, measured as its ability to cleave its substrate peptide IETD (Figure 4C and Supplementary Figure S2B). Caspase-8 activity starts to be detectable at 12 h of expression, according to the appearance of its processing products, peaks between 13 and 14 h, and dramatically decreases at 16 h, consistently with the decrease of its expression levels (Supplementary Figure S2). Src expression significantly delays Caspase-8 activation at 12 and at 13 h of induction (Figure 4C and Supplementary Figure S2B). These experiments allowed us to conclude that tyrosine phosphorylation impairs Caspase-8 activity in this system.
Figure 4.
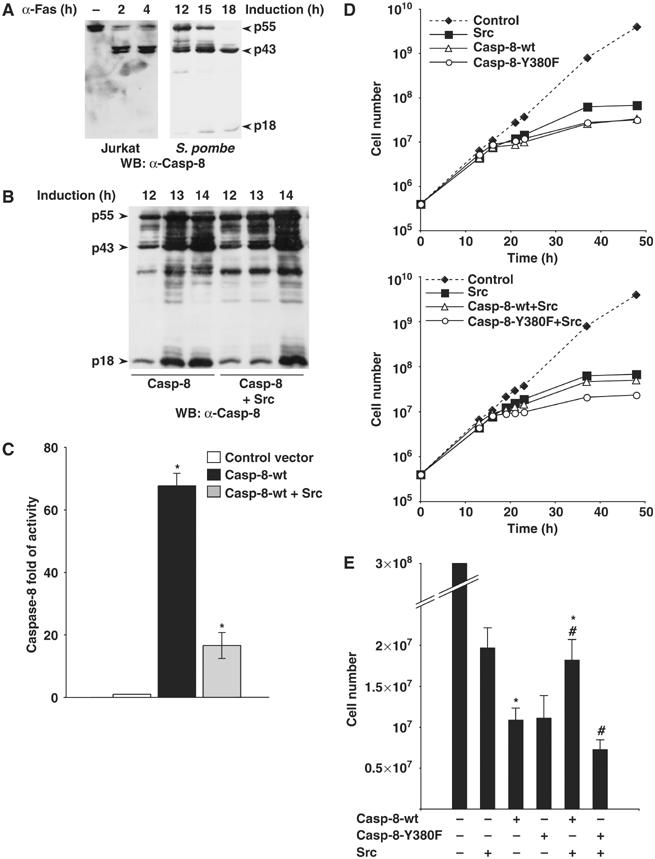
Src kinase phosphorylates Caspase-8 on Tyr380 and modulates its processing and activity in yeast. (A) Protein extracts from Jurkat cells stimulated to undergo apoptosis with anti-Fas antibodies, and protein extracts from S. pombe at different times of Caspase-8 expression induction, have been separated by SDS–PAGE and Caspase-8 revealed by immunoblotting with specific antibodies. The arrows point to the entire protein, p55, as well as to the processing products p43 and p18. (B) Extracts from S. pombe transfected with Caspase-8-wt in the presence or not of Src, at different times of induction, have been processed for SDS–PAGE and immunoblotting with specific antibodies. (C) Caspase-8 activity from S. pombe extracts at 13 h of induction was measured by the hydrolysis of the Caspase-8 substrate Ac-IETD-AMC. The differences between Caspase-8-wt±Src (*) are statistically significant by t-test (*P=0.02). (D) Growth curves of S. pombe cells expressing Src from the inducible nmt1 promoter of the pRSP vector and Caspase-8 wt or Y380F from the inducible nmt1 promoter of the pNU vector. The y-axis is logarithmic. (E) The histogram represents the number of cells, at 36 h after induction of expression of proteins. Each bar represents the mean value±s.d. (n=8). The differences in the cell number between Caspase-8-wt±Src (*) and between Caspase-8-wt+Src and Caspase-8-Y380F+Src (#) are statistically significant by t-test (*P=0.02, #P=0.0003).
Caspase-8-wt and Caspase-8-Y380F are equally toxic (Figure 4D and E) indicating that Tyr380Phe mutation does not affect per se Caspase-8 activity. Interestingly, despite its own toxicity, Src coexpression slightly relieved the yeast from Caspase-8 toxicity, suggesting that phosphorylation modulates Caspase-8 activity. Src protective effect requires the phosphorylation of Tyr380 of Caspase-8, since Src coexpression failed to ameliorate the Caspase-8-Y380F toxicity (Figure 4D). Overall, this system allowed us to clarify that Src directly phosphorylates and inactivates Caspase-8 in vivo (data not shown and Figure 4).
Tyrosine phosphorylation modulates Caspase-8 processing and activity in mammalian cells
Fas-receptor stimulation leads to Caspase-8 recruitment to the DISC, dimerization and processing (Boatright et al, 2003; Chang et al, 2003; Donepudi et al, 2003).
Our observation that Caspase-8 activity is downregulated by Src expression in yeast, where Fas-receptor is absent, suggests that Src effect on Caspase-8 activity does not involve its recruitment to the DISC. It has been previously shown that incubation of cell extracts in the presence of Sodium Citrate triggers Procaspase-8 dimerization and activation (Boatright et al, 2003). To investigate whether tyrosine phosphorylation could modulate Procaspase-8 dimerization, protein extracts from HeLa cells transfected with SrcY527F or with an empty vector as a control, were incubated in the presence or in the absence of Sodium Citrate to induce Procaspase-8 dimerization in the presence of biotinyl-VAD-fmk, which labels caspases' active sites. Labeled active Procaspase-8 dimers were then immunoprecipitated with agarose-conjugated streptavidin beads and analysed by Western blot with anti-Caspase-8 antibody. This assay allows the detection of active Procaspase-8, which correlates with its ability to dimerize. Src activity does not affect the amount of biotinyl-VAD-fmk-labelled-Procaspase-8, activated by Sodium Citrate, suggesting that tyrosine phosphorylation does not modulate dimerization. Using our monoclonal phospho-specific antibody that selectively recognizes Tyr380 phosphorylated Caspase-8 (Supplementary Figure S1), we could show that indeed phosphorylated Caspase-8 is able to dimerize (Figure 5A).
Figure 5.

Phosphorylation on Tyr380 modulates the activity of Caspase-8 and protects human cells from Fas-induced apoptosis. (A) HeLa cells were transiently transfected with SrcY527F, or with empty vector, and Procaspase-8 dimerization was triggered by incubation of protein extracts in the presence of Sodium Citrate. Active dimerized Procaspase-8 was revealed by Biotin-VAD-fmk labeling, immunoprecipitation with agarose-conjugated streptavidin beads and immunoblotting. (B) HeLa cells transfected as indicated were stimulated to undergo apoptosis with 250 ng/ml α-Fas plus 1 μg/ml cycloheximide for 2.5 and 5 h. Total lysates were separated by SDS–PAGE and immunoblotted with specific anti-Caspase-8 antibodies. (C) Active Caspase-8 from 107cells, transfected as in (A) and stimulated with 500 ng/ml α-Fas plus 1 μg/ml cycloheximide for 4 h, was labeled with Biotin-VAD-fmk, immunoprecipitated with agarose-conjugated streptavidin beads and immunoblotted as indicated. (D) Caspase-8-deficient Jurkat I9.2 cell line was transiently transfected with Caspase-8-wt or Caspase-8-Y380F in the presence or not of SrcY527F, together with pEGFP-N1-spectrin. At 12 h after transfection, cells were incubated with 500 ng/ml anti-Fas antibodies for 8 h. Apoptosis was measured by flow-cytometry upon propidium iodide staining of the GFP-positive population. The data are presented as the mean value±s.d. of five independent experiments. The differences between Caspase-8-wt+Src and Caspase-8 (*), and between Caspase-8-wt+Src and Caspase-8-Y380F+Src (#) are statistically significant by t-test (*P=0.04, #P=0.01).
We therefore investigated whether tyrosine phosphorylation could affect Caspase-8 processing upon Fas-receptor stimulation. In this pathway, processing is essential to get a stable active Caspase-8 tetramer and to allow its release from the DISC and subsequent cleavage of cytoplasmic substrates, such as executioner caspases. Extracts from Fas-stimulated HeLa cells transfected with SrcY527F or with the empty vector control, were processed for immunoblot analysis using an anti-Caspase-8 antibody raised against the p18 subunit. The expression of SrcY527F results in the delayed accumulation of the p18 subunit (Figure 5B). The appearance of the p18 subunit can be evaluated as a marker for Caspase-8 activation. Indeed, Src expression reduces the ability of the p18, generated upon Fas-receptor stimulation, to bind biotinyl-VAD-fmk (Figure 5C). Overall, these experiments support the idea that Src kinase activity interferes with Caspase-8 processing and activation.
Phosphorylation on Tyr380 impairs Caspase-8 activation upon Fas receptor stimulation
To investigate the effect of Tyr380 phosphorylation on Caspase-8 activity, we chose the Jurkat Caspase-8−/− cell line. These cells do not express Caspase-8 and therefore do not undergo apoptosis upon Fas-receptor stimulation, yet the reconstitution of Caspase-8 expression restores the sensitivity to Fas-induced apoptosis (Juo et al, 1998). Since Caspase-8 overexpression triggers its own activation, which induces cell death per se, these experiments require the expression of very low levels of transfected Caspase-8, which allow the detection of Fas-induced apoptosis. Caspase-8-wt and Caspase-8-Y380F restore Fas sensitivity to the same extent, confirming that the substitution of Tyr380 with Phe does not affect per se Caspase-8 function. Importantly, coexpression of the constitutively active Src, SrcY527F, suppressed Fas-induced apoptosis in cells reconstituted with Caspase-8 wt, but not in those reconstituted with Caspase-8-Y380F, indicating that Src-mediated protection requires Caspase-8 Tyr380 phosphorylation (Figure 5D) and suggesting that the phosphorylation of Tyr380 modulates Caspase-8 activity and function.
EGF triggers endogenous Caspase-8 phosphorylation and counteracts Fas-induced apoptosis
Endogenous Src kinase activity is tightly regulated and is induced upon different stimuli. To further investigate whether the activation of endogenous Src could trigger Caspase-8 phosphorylation, HeLa cells were treated with EGF, which directly activates Src kinase (Figure 6A). Immunoblotting with anti-phosphotyrosine antibodies on immunoprecipitated Caspase-8 showed that EGF treatment triggered Caspase-8 phosphorylation (Figure 6A). We further asked whether Caspase-8 phosphorylation could affect Fas sensitivity in this context. Exposure to EGF caused a significant delay in Fas-induced apoptosis of HeLa cells (Figure 6B). Interestingly, EGF failed to protect cells stably transfected with a kinase defective version of Src (SrcY527F-kin−) from Fas-induced apoptosis, suggesting that the antiapoptotic effect of EGF relies on Src kinase activity (Figure 6B) and, most likely, on Caspase-8 phosphorylation.
Figure 6.
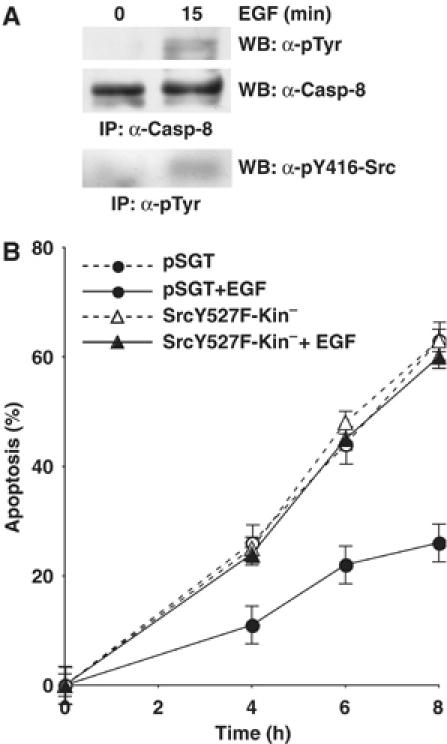
EGF triggers Caspase-8 tyrosine phosphorylation and protects cells from Fas-induced apoptosis. (A) HeLa cells were serum-deprived for 24 h and then treated with 100 ng/ml EGF for 15 min. Immunoprecipitated Caspase-8 was separated by SDS–PAGE gel and analyzed by immunoblotting with anti-phosphotyrosine and with anti-Caspase-8 antibodies. Src activation was revealed by immunoblotting with an antibody that selectively recognizes active Src, upon immunoprecipitation with anti-phosphotyrosine antibodies (B) HeLa cells stably transfected with Src-Y527F-Kin− or with empty vector as control have been serum starved for 24 h and stimulated to undergo apoptosis with 500 ng/ml anti-Fas antibody, 1 μg/ml CHX, in the presence or not of 100 ng/ml EGF. Apoptotic cells have been identified by Hoechst-stained fragmented nuclei. The rate of apoptosis has been calculated at different times of stimulation. The data are presented as the mean value±s.d. of six independent experiments.
Caspase-8 is phosphorylated on Tyr380 in colon cancer
We reported that the activation of Src in a physiological situation such as EGF treatment triggers Caspase-8 phosphorylation. We therefore asked whether Caspase-8 may be tyrosine phosphorylated in those pathological situations where Src kinase is aberrantly upregulated such as different types of tumors (Frame, 2002). We speculated that tyrosine phosphorylation of Caspase-8 may represent a new mechanism through which transformed cells evade apoptosis and promote cancer. We analyzed the profile of Caspase-8 in colonic adenocarcinomas from 23 patients. While the levels of Src protein in cancer mucosa and in the adjacent normal mucosa are comparable (Figure 7A), the kinase activity of Src is significantly increased in about 80% of the tumors (Figure 7B and C, Supplementary Figure S3 and Supplementary Table), according to previous reports from other laboratories (Allgayer et al, 2002). More interestingly, we were able to immunoprecipitate Caspase-8 with anti-phosphotyrosine antibodies, selectively from cancer mucosa (Figure 7B). In the reverse experiment, we could detect tyrosine-phosphorylated Caspase-8 upon immunoprecipitation with anti-Caspase-8 antibodies and immunoblotting with anti-phosphotyrosine antibodies (Figure 7C). We revealed the presence of tyrosine-phosphorylated Caspase-8 in 19 out of 23 analyzed patients (Supplementary Table). Using our phospho-specific antibody, which selectively recognizes phosphorylated Tyr380 (Supplementary Figure S1), we were able to show that Caspase-8 is phosphorylated on Tyr380 in primary colon cancer (Figure 7D, Supplementary Figure S3 and Supplementary Table).
Figure 7.
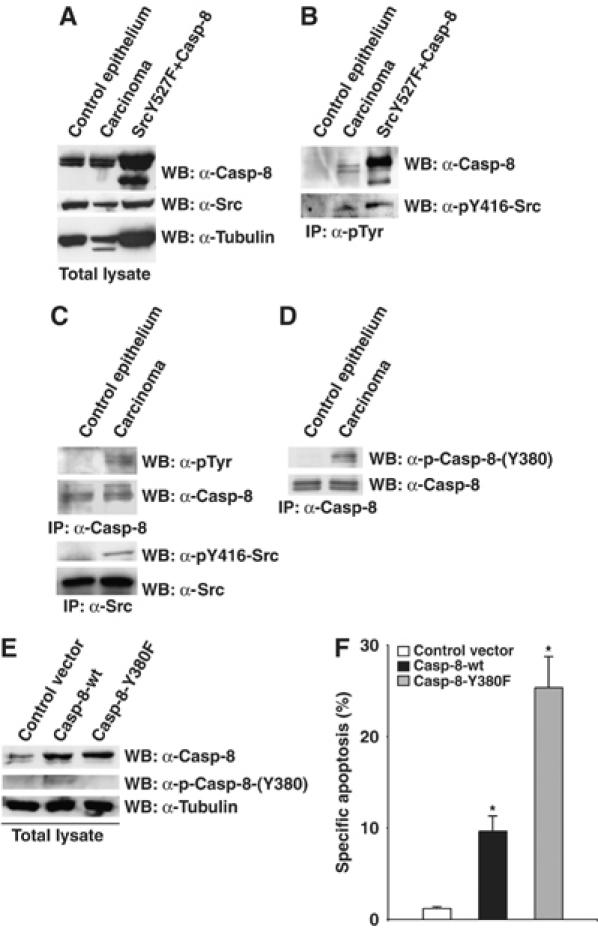
Caspase-8 is phosphorylated on Tyr380 in colon cancer. (A) Protein extracts were prepared from human colorectal carcinoma and corresponding normal samples. Total proteins were separated by SDS–PAGE and immunoblotted with specific antibodies. (B) The pool of tyrosine phosphorylated proteins was immunoprecipitated with anti-phosphotyrosine antibodies and Caspase-8 and active Src detected by immunoblotting with specific antibodies. (C) Caspase-8 was immunoprecipitated and tyrosine phosphorylation was revealed by immunoblotting with anti-phosphotyrosine antibodies. Src was immunoprecipitated and its activity was revealed by immunoblotting with specific antibodies. (D) Caspase-8 was immunoprecipitated with anti-Caspase-8 antibodies and immunoblotted with phospho-Casp8-(Y380) antibody. (E) Extracts from DLD-1/TRAIL-R cells transiently transfected with the indicated constructs were immunoblotted as shown. (F) DLD-1/TRAIL-R cell line was transiently transfected with Caspase-8-wt or Caspase-8-Y380F together with pEGFP-N1-spectrin. At 24 h after transfection, cells were incubated with 500 ng/ml anti-Fas plus 1 μg/ml cycloheximide for 3 h. Apoptosis was measured by flow-cytometry upon propidium iodide staining of the GFP-positive population. The data are presented as the mean value±s.d. of three independent experiments (*P=0.03).
To further investigate whether Caspase-8 phosphorylation on Tyr380 could modulate Caspase-8 activity in human colon cancer, we took advantage of a human colon cancer cell line previously selected for being resistant to tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced apoptosis, named DLD1/TRAIL-R (Zhang et al, 2005). These cells have been shown to express low levels of Caspase-8, which are not sufficient to trigger TRAIL-induced apoptosis. Reconstitution of Caspase-8 expression is sufficient to sensitize back these cells to TRAIL (Zhang et al, 2005). We observed that DLD1/TRAIL-R cells are also resistant to Fas-induced apotosis (Figure 7F). We reconstituted these cells with Caspase-8-wt or Caspase-8-Y380F (Figure 7E). Immunoblotting with our phospho-specific antibody revealed that Caspase-8-wt but not its unphosphorylatable counterpart is phosphorylated on Tyr380 in these cells, suggesting the presence of endogenous active Src (Figure 7E). Interestingly, reconstitution of Caspase-8 expression sensitize back these cells to Fas-induced apoptosis. However, cells reconstituted with Caspase-8-wt, phosphorylatable on Tyr380, are significantly less sensitive to Fas than cells reconstituted with the unphosphorylatable mutant, Caspase-8-Y380F (Figure 7F), supporting the idea that tyrosine phosphorylation impairs Caspase-8 activity in colon cancer.
Discussion
We are investigating the crosstalk between non-receptor tyrosine kinases and caspases and whether these two families of proteins can modulate each other's function through their enzymatic activities. Surprisingly, however, despite the fact that tyrosine kinases play a fundamental role in cell survival and growth, no caspases have been identified as substrates of tyrosine kinases. Nevertheless, inhibition of tyrosine kinases triggers apoptosis in many experimental systems (Griffiths et al, 2004) and the tyrosine phosphatase SHP-1 sensitizes cells to Fas-induced cell death (Su et al, 1995; Daigle et al, 2002).
Caspase-8 is absolutely required for Fas-induced apoptosis (Juo et al, 1998). Therefore, we tested whether non-receptor tyrosine kinases impinge on Fas-signaling directly targeting Caspase-8. Here we show that Src kinase directly phosphorylates Caspase-8. Src activity triggers endogenous Caspase-8 tyrosine phosphorylation and protects cells from Fas-induced apoptosis, suggesting that tyrosine phosphorylation directly modulates Caspase-8 activity and function. Using an enzymatic assay for Caspase-8 activity, we could show that the expression of a constitutively active Src severely impairs endogenous Caspase-8 activation, similarly to what was observed upon expression of a potent inhibitor of caspases such as CrmA. Moreover, the expression of a constitutively active Src delays Procaspase-8 processing and the following accumulation of its cleavage products. These events normally occur upon Fas-stimulation as the final result of Procaspase-8 recruitment to the DISC, dimerization and activation. Since Fas-induced apoptosis is strictly dependent on Caspase-8 activation, and Caspase-8 activation upon Fas-stimulation ends with its autoprocessing, the observation that Src kinase activity downregulates Caspase-8 cleavage, suggests that it directly impinge on Caspase-8 activation.
Mass spectrometry and point mutagenesis on immunoprecipitated Caspase-8 from cells cotransfected with Caspase-8 and an active Src identified a single phosphorylation on Tyr380. Interestingly, a database search using ScanSite (Songyang et al, 1995) identified this site as an optimal putative phosphorylation site for Fgr kinase, a member of the Src family kinases.
The generation of Caspase-8-Y380F mutant that cannot be phosphorylated by Src kinase allowed us to test the significance of this phosphorylation on Caspase-8 activity and function. Remarkably, Tyr380Phe mutation per se does not affect the function of Caspase-8, neither in S. pombe nor in the reconstituted Caspase-8-deficient mammalian cells. However, Src kinase expression relieves yeast from Caspase-8 but not from Caspase-8-Y380F toxicity, indicating that Tyr 380 phosphorylation modulates Caspase-8 activity. Moreover, the experiments on the reconstituted Caspase-8-deficient mammalian cells show that Tyr 380 phosphorylation modulates Fas-induced apoptosis.
To further investigate the biological relevance of this phosphorylation, we asked whether Caspase-8 might be tyrosine phosphorylated in any physiological or pathological context where Src kinase activity is upregulated. We observed that the activation of endogenous Src kinase activity upon EGF receptor stimulation (Bromann et al, 2004) triggers Caspase-8 tyrosine phosphorylation. Moreover, Src kinase activity is necessary for the antiapoptotic effect of EGF on Fas-induced cell death. This finding, together with the data obtained in yeast and in Caspase-8-deficient mammalian cells, allows speculating that EGF protection relies on Src's ability to phosphorylate and modulate Caspase-8 activity.
Src family kinases are upregulated in epithelial and non-epithelial tumors (Frame, 2002; Summy and Gallick, 2003; Warmuth et al, 2003). Significantly, we were able to detect tyrosine phosphorylated Caspase-8 in 80% of human colon tumors. This event matches the observation that Src kinase activity is also upregulated in the same percentage of these tumors (Allgayer et al, 2002). Moreover, we could show that Caspase-8 is specifically phosphorylated on Tyr380 in colon cancer. This finding, together with the data showing that phosphorylation on this residue downregulates Caspase-8 activity, allows us to propose that Src may contribute to cancer formation and development through the downregulation of the Caspase-8-dependent apoptotic machinery. Interestingly, several cancers show genetic alterations that lead to a lack of Caspase-8 activity, supporting the idea that the impairment of Caspase-8 function contributes to cancer development (Teitz et al, 2000; Harada et al, 2002; Kim et al, 2003; Ashley et al, 2005; Soung et al, 2005). Many tumors are resistant to death receptor-induced apoptosis as well as to other treatments that trigger Caspase-8 activation (Nicholson, 2000; Igney and Krammer, 2002), and carcinoma cells can be sensitized to TRAIL by treatment with chemotherapeutic drugs that enhance Caspase-8 activation (Ganten et al, 2004). Interestingly, inhibition of Src sensitizes human colon cancer cell lines to Fas-induced apoptosis (Griffiths et al, 2004). Importantly, we could show that Caspase-8 phosphorylation on Tyr380 contributes to Fas resistance in colon cancer cells. These observations suggest that Caspase-8 activity plays an important role also in cancer resistance to therapy.
Tyr380 is conserved through evolution in other mammals, such as chimp and dog, but not in rat and mouse. However, it is conserved also in the putative linker region of other species such as chicken, zebrafish and Xenopus. This observation suggests that Src may not trigger Caspase-8 tyrosine phosphorylation in rodents and that this circuit may not be functional in mice and rats. Importantly, despite the overall similarities between humans and mice transformation and carcinogenesis, an emerging body of evidence indicates that there are fundamental differences in how the process of tumorigenesis occurs in the two species (Rangarajan and Weinberg, 2003). Overall, humans are more sensitive to epithelial tumors while mice preferentially develop mesenchymal tumors. Moreover, mice are more resistant to the development of colon cancer (Rangarajan and Weinberg, 2003). These differences point to the evolution of species-specific mechanisms that differentially control tumorigenesis, which may account for the absence of Caspase-8 tyrosine phosphorylation in rodents.
Tyr380 lies in the linker region between the large and the small subunits. The p18 and p10 subunits are separated by a linker peptide also in other caspases, such as Caspase-1, 2, 4, 5 and 6 (Cohen, 1997). However, only Caspase-8 shows the presence of a Tyr residue in this region.
No stuctural data are available for Procaspase-8, therefore the conformation and the role of the linker in the control of caspase activation remains speculative. More importantly, we lack any structural information to be able to predict how phosphorylation on Tyr380 modulates Caspase-8 proapoptotic function. Tyrosine phosphorylation may modulate one of the steps of Caspase-8 activation, such as DISC recruitment, dimerization, activation, processing and release of the active tetramer. Our experimental data support the hypothesis that phosphorylation on Tyr380 mainly modulates Caspase-8 activity and function affecting its processing. Phosphorylation on Tyr380 may also drive new protein–protein interactions that either change the conformation of Caspase-8 preventing its activation, or sequester it far from its substrates, impinging on its apoptotic function. Further experiments will address this point. Moreover, it will be interesting to investigate the possibility that this event occurs also in nonapoptotic responses where Caspase-8 activation has been involved, such as lymphocyte differentiation (Su et al, 2005).
Overall, our findings provide a direct link between Src kinase and Caspase-8 activity modulation and suggest that the inhibition of Src kinase activity might be used to sensitize human carcinomas to treatments that exploit death receptor-induced apoptosis.
Materials and methods
DNA constructs
pSGT-SrcY527F, pSGT-SrcY527F/K295M(kin−), pSGT-Src-ΔSH3 pRSP-Src, pSGT-Abl-PP, Bcr-Abl and c-Jun were previously described (Barilà et al, 2003). All Src DNAs were generated from chicken Src sequence (c-Src). All human Caspase-8 constructs were derived from pcDNA3-Caspase-8-HA, generously provided from R Beyaert (Ghent University, Belgium). Briefly, pcDNA3-Myc-Caspase-8 was obtained by PCR with specific oligonucleotides designed to insert Caspase-8 sequence into pCDNA3-Myc, digested with BamHI and XbaI, in frame with two Myc tag at the 5′. pEGFP-CrmA was generated from pHD1.2-CrmA (provided by J Yuan, Harvard Medical School, Boston, USA) by PCR amplification with specific oligonucleotides designed to subclone the fragment into pEGFP-C3 (Clontech) using HindIII and ApaI restriction sites. pNU-Caspase-8 was generated by PCR, with specific oligonucleotides designed to subclone Caspase-8 sequence into pNU vector digested with SpeI–BamHI. pCDNA3-Myc-Caspase-8-Y380F and pNU-Caspase-8-Y380F were generated using the QuickChange site-directed mutagenesis kit (Stratagene) using pCDNA3-Myc-Caspase-8 and pNU-Caspase-8, respectively, as templates. pEGFP-N1-Spectrin was kindly provided by A Beavis and R Kalejta (Princeton University, USA).
Antibodies and other reagents
The following antibodies and reagents were used: anti-Caspase-8 (clone 5F7, MBL), anti-phosphotyrosine (clone 4G10, UBI), anti-Src monoclonal antibodies 2–17 (Microbiological Associates), phospho-Src-Y416 (Cell Signaling), EGF human recombinant (UBI), anti-Fas IgM monoclonal antibody (CH11; UBI), Hoechst 33342 (Molecular Probes) A mouse monoclonal phosphospecific antibody, p-Casp-8-(Y380), was generated (EMBL, Mouse Monoclonal Antibodies Facility) against a Caspase-8 phosphopeptide, CGEQ*YLEMDLSSPQTR, where * represents the phosphotyrosine (Sigmagenosys).
Cell culture and transfections
HEK293 and HeLa cells were maintained in DMEM medium supplemented with 10% fetal bovine serum and transfected with the calcium phosphate precipitation method as previously described (Barilà et al, 2003). Wild-type Jurkat cells and Caspase-8-deficient Jurkat mutant, I9.2 (ATCC), were cultured in RPMI 1640 medium with 10 mM HEPES, 1.0 mM sodium pyruvate, 10% fetal bovine serum. Jurkat I9.2 were transiently transfected using Lipofectamine 2000 (Life Technologies) essentially following the manufacturer's intruction. Briefly, 1 × 106 cells were transfected in 1 ml with 6 μl LipofectAMINE 2000 and 4 μg of total DNA (0.5 μg pEGFP-N1-Spectrin, 1 μg pcDNA Caspase-8-wt, 1 μg pcDNA Caspase-8-Y380F, 2.5 μg pSGT Src Y527F), and stimulated to undergo apoptosis with 500 ng/ml of anti-Fas mAb. DLD-1/TRAIL-R, kindly provided by B Fang (University of Texas, USA), were grown in DMEM, supplemented with 10% fetal bovine serum, transfected as described for Jurkat cells and stimulated to undergo apoptosis with 500 ng/ml of anti-Fas mAb plus 1 μg/ml cycloheximide for 3 h.
Analysis of apoptosis
Transfected or nontransfected HeLa cells were serum-deprived for 24 h. Cells were then treated with 250–500 ng/ml of anti-Fas mAb plus 1 μg/ml cycloheximide, in the presence or absence of 100 ng/ml EGF. Apoptosis was determined at different times after anti-Fas treatment by counting Hoechst-stained fragmented nuclei among GFP-positive cells. Blind counting of at least 200 cells were performed for each group.
Fas-stimulated Jurkat and DLD1/TRAIL-R cells were analyzed for DNA fragmentation by FACS analysis. Apoptosis was quantitated by propidium iodide nuclear staining within the electronically gated GFP-positive population, using a FACScan (Becton Dickinson).
Specific apoptosis was determined as follows: (% of apoptotic cells with anti-Fas−% of apoptotic cells without anti-Fas)/(100−% of apoptotic cells without anti-Fas).
Immunoblotting and immunoprecipitation
Cell extracts were prepared in IP buffer (50 mM Tris–HCl (pH 7.5), 250 mM NaCl, 1% NP-40, 5 mM EDTA, 5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 25 mM NaF, 1 mM orthovanadate, 10 μg/ml TPCK, 5 μg/ml TLCK, 1 μg/ml leupeptin, 10 μg/ml soybean trypsin inhibitor, 1 μg/ml aprotinin) (Barilà et al, 2003). For immunoblotting, 50–100 μg of protein extract were separated by SDS–PAGE, blotted onto nitrocellulose membrane and detected with specific antibodies. For immunoprecipitation, protein extracts prepared as above were incubated for 1–2 h with specific antibodies previously conjugated to proteinA-sepharose (Amersham). Immunocomplex were then resolved and analysed by SDS–PAGE. All immunoblots were revealed by ECL (Amersham).
Mass spectrometric analysis (MS)
Bands corresponding to Caspase-8 were excised from the gel, subsequently reduced, alkylated and digested overnight with bovin trypsin. Proteins were unambiguously identified by MALDI-TOF peptide mass mapping. 1 μl of the supernatant of the digestion was loaded onto the Maldi target using the dried droplet technique and α-cyano-4-hydroxycinnamic acid (HCCA) as matrix. Maldi-MS measurements were performed on a Voyager-DE STR (Applied Biosystems) time of flight (TOF) mass spectrometer and processed via the Data Explorer software. Phosphorylated peptides were analyzed by Tandem MS experiments performed on a Q-Star pulsar (QqTof hybrid system from PE SCIEX Instrument, Toronto, Canada).
Phosphopeptides were purified with Immobilized Metal Affinity Chromatography (IMAC). IMAC was performed using the Phosphopeptide Isolation Kit (Pierce Biotechnology) according to the manufacturer's instructions. Phosphopeptides, detected by MALDI-TOF MS, were confirmed by alkaline phosphatase treatment on target.
Yeast strains and culture conditions
All yeast studies employed the S. pombe strain SP200 (h−sleu1-32 ura4 ade2-10). Growth conditions, media and induction were according to Superti-Furga et al (1993). Cells were grown either in full medium with addition of adenine (YEA) or minimal medium containing adenine (PMA) and leucine or uracil where appropriate. To repress the nmt1 promoter, cells were kept in medium containing 4 μM thiamine. Transformation was carried out by the lithium acetate (LiAc) method as described. Protein extracts from S. pombe cells were produced as described previously (Superti-Furga et al, 1993).
Caspase-8 activity assays
To determine Caspase-8 activity in HeLa extracts, 24 h after transfection, cells were induced to undergo apoptosis with 250 ng/ml of anti-Fas mAb plus 1 μg/ml cycloheximide. Proteins were extracted from apoptotic cells in protein IP buffer. Ac-IETD-pNA colorimetric assay was performed at 37°C in 96-well plate, in 100 μl assay buffer (50 mM Hepes pH 7.4, 100 mM NaCl, 0.1% CHAPS, 1 mM EDTA, 10% glycerol, 10 nM DTT) containing 100 μg protein extract. Reaction was started by the addition of 200 μM Ac-IETD-pNA and monitored by reading the absorbance at 405 nm. Absorbance over the linear portion of the curve was converted in nmol of substrate hydrolyzed/min using an extinction coefficient for p-nitroaniline of 10 500 M-1 cm-1.
Pombe protein extracts were assayed for Caspase-8 activity using Ac-IETD-AMC as a substrate at 37°C in 200 μl assay buffer (20 mM Tris, pH 7.4, 0.1 M NaCl, 10% sucrose, 0.1% CHAPS, 10 mM DTT) containing 250 μg protein extract. Reaction was started by the addition of 10 μM Ac-IETD-AMC. Cleavage of the substrate as a function of time was monitored reading the absorbance at 460 nm upon excitation at 390 nm.
The enzymatic activity was determined from the linear portion of the curve.
Caspase-8 dimerization assay and BiotinVAD binding
Protein extracts from HeLa cells were incubated for 45 min at 37°C with or without 0.7 M Na citrate to induce Caspase-8 dimerization in the presence of 10 μM biotinyl-VAD-fmk, which labels caspases' active sites. Labeled active Procaspase-8 dimers were then immunoprecipitated with agarose-conjugated streptavidin beads and analysed by Western blot.
For Biotin-VAD labeling of apoptotic cell extracts, proteins were extracted in IP buffer and labeled with 10 μM biotinyl-VAD-fmk for 45 min at 37°C. Labeled proteins were then immunoprecipitated with agarose-conjugated streptavidin beads and analysed by Western blot.
Analysis of Caspase-8 tyrosine phosphorylation in human colon cancer
Colorectal adenocarcinoma and corresponding normal tissue were obtained from 23 patients who underwent surgical resection. Each sample was rapidly frozen in liquid nitrogen and homogenized on ice, in lysis buffer (IP buffer plus 0.1%Triton X-100).
Statistical methods
All data were analyzed and presented as mean±s.d. (n<10). The significance of differences between populations of data were assessed according to the Student's two-tailed t-test with a level of significance of at least P<0.05 (alpha conventionally equal to 0.05). This analysis arises in the problem of estimating the mean of a normally distributed population when the sample size is small.
Supplementary Material
Supplementary Information
Acknowledgments
We acknowledge R Beyaert, P Vandenabeele, J Yuan, A Beavis, R Kalejta, B Fang, D Nicholson, C Pop and G Salvesen for kindly providing reagents and suggestions, O Hantschel and MG di Bari for helpful discussion and critical reading of the manuscript, D Serio for technical assistance and N Ventura for helping with the FACs analysis. DB is an Assistant Telethon Scientist and is supported by the Italian Telethon Grant (TCP00061), SC has been supported by the Italian Telethon Foundation, VS and AR have been supported by the Italian Foudation for Cancer Research (FIRC). This work has been supported by grants from the Italian Telethon Foundation (TCP00061), from the Italian Association for Cancer Research (AIRC) and from the Italian Compagnia di San Paolo-Imi Bank Foundation to DB. This work was also supported by a grant from AIRC to RT.
References
- Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR (2003) Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol 5: 647–654 [DOI] [PubMed] [Google Scholar]
- Allgayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE (2002) Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer 94: 344–351 [DOI] [PubMed] [Google Scholar]
- Alvarado-Kristensson M, Melander F, Leandersson K, Ronnstrand L, Wernstedt C, Andersson T (2004) p38-MAPK signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J Exp Med 199: 449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley DM, Riffkin CD, Muscat AM, Knight MJ, Kaye AH, Novak U, Hawkins CJ (2005) Caspase 8 is absent or low in many ex vivo gliomas. Cancer 104: 1487–1496 [DOI] [PubMed] [Google Scholar]
- Barilà D, Rufini A, Condò I, Ventura N, Dorey K, Superti-Furga G, Testi R (2003) Caspase-dependent cleavage of c-Abl contributes to apoptosis. Mol Cell Biol 23: 2790–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard H, Kodandapani L, Mittl PR, Di Marco S, Krebs JF, Wu JC, Tomasselli KJ, Grutter MG (1999) The three-dimensional structure of caspase-8: an initiator enzyme in apoptosis. Structure 7: 1125–1133 [DOI] [PubMed] [Google Scholar]
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS (2003) A unified model for apical caspase activation. Mol Cell 11: 529–541 [DOI] [PubMed] [Google Scholar]
- Bromann PA, Korkaya H, Courtneidge SA (2004) The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23: 7957–7968 [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282: 1318–1321 [DOI] [PubMed] [Google Scholar]
- Chang DW, Xing Z, Capacio VL, Peter MP, Yang X (2003) Interdimer processing mechanism of procaspase-8 activation. EMBO J 22: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326 (Part 1): 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle I, Yousefi S, Colonna M, Green DR, Simon HU (2002) Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signaling in neutrophils. Nat Med 8: 61–67 [DOI] [PubMed] [Google Scholar]
- Donepudi M, Sweeney AM, Briand C, Grutter MG (2003) Insights into the regulatory mechanism for caspase-8 activation. Mol Cell 11: 543–549 [DOI] [PubMed] [Google Scholar]
- Frame MC (2002) Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 1602: 114–130 [DOI] [PubMed] [Google Scholar]
- Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC, Krueger A, Weigand MA, Grosse-Wilde A, Stremmel W, Krammer PH, Walczak H (2004) Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell Death Differ 11 (Suppl 1): S86–S96 [DOI] [PubMed] [Google Scholar]
- Griffiths GJ, Koh MY, Brunton VG, Cawthorne C, Reeves NA, Greaves M, Tilby MJ, Pearson DG, Ottley CJ, Workman P, Frame MC, Dive C (2004) Expression of kinase-defective mutants of c-Src in human metastatic colon cancer cells decreases Bcl-xL and increases oxaliplatin- and Fas-induced apoptosis. J Biol Chem 279: 46113–46121 [DOI] [PubMed] [Google Scholar]
- Harada K, Toyooka S, Shivapurkar N, Maitra A, Reddy JL, Matta H, Miyajima K, Timmons CF, Tomlinson GE, Mastrangelo D, Hay RJ, Chaudhary PM, Gazdar AF (2002) Deregulation of caspase 8 and 10 expression in pediatric tumors and cell lines. Cancer Res 62: 5897–5901 [PubMed] [Google Scholar]
- Hunter T (2000) Signaling-2000 and beyond. Cell 100: 113–127 [DOI] [PubMed] [Google Scholar]
- Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2: 277–288 [DOI] [PubMed] [Google Scholar]
- Juo P, Kuo CJ, Yuan J, Blenis J (1998) Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol 8: 1001–1008 [DOI] [PubMed] [Google Scholar]
- Kim HS, Lee JW, Soung YH, Park WS, Kim SY, Lee JH, Park JY, Cho YG, Kim CJ, Jeong SW, Nam SW, Kim SH, Lee JY, Yoo NJ, Lee SH (2003) Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology 125: 708–715 [DOI] [PubMed] [Google Scholar]
- Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME (1997) FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J 16: 2794–2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM (1996) FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85: 817–827 [DOI] [PubMed] [Google Scholar]
- Nicholson DW (2000) From bench to clinic with apoptosis-based therapeutic agents. Nature 407: 810–816 [DOI] [PubMed] [Google Scholar]
- Peter ME (2004) The flip side of FLIP. Biochem J 382: e1–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Weinberg RA (2003) Opinion: comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer 3: 952–959 [DOI] [PubMed] [Google Scholar]
- Silke J, Ekert PG, Day CL, Hawkins CJ, Baca M, Chew J, Pakusch M, Verhagen AM, Vaux DL (2001) Direct inhibition of caspase 3 is dispensable for the anti-apoptotic activity of XIAP. EMBO J 20: 3114–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Carraway KLI, Eck MJ, Harrison SC, Feldman RA, Mohammadi M, Schlessinger J, Hubbard SR, Smith DP, Eng C, Lorenzo MJ, Ponder BAJ, Mayer BJ, Cantley LC (1995) Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature 373: 536–539 [DOI] [PubMed] [Google Scholar]
- Soung YH, Lee JW, Kim SY, Sung YJ, Park WS, Nam SW, Kim SH, Lee JY, Yoo NJ, Lee SH (2005) Caspase-8 gene is frequently inactivated by the frameshift somatic mutation 1225_1226delTG in hepatocellular carcinomas. Oncogene 24: 141–147 [DOI] [PubMed] [Google Scholar]
- Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S, Lenardo M (2005) Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science 307: 1465–1468 [DOI] [PubMed] [Google Scholar]
- Su X, Zhou T, Wang Z, Yang P, Jope RS, Mountz JD (1995) Defective expression of hematopoietic cell protein tyrosine phosphatase (HCP) in lymphoid cells blocks Fas-mediated apoptosis. Immunity 2: 353–362 [DOI] [PubMed] [Google Scholar]
- Summy JM, Gallick GE (2003) Src family kinases in tumor progression and metastasis. Cancer Metast Rev 22: 337–358 [DOI] [PubMed] [Google Scholar]
- Superti-Furga G, Fumagalli S, Koegl M, Courtneidge SA, Draetta G (1993) Csk inhibition of c-src activity requires both the SH2 and SH3 domains of Src. EMBO J 12: 2625–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM, Kidd VJ (2000) Caspase 8 is deleted or silenced preferentially in chidhood neuroblastomas with amplification of MYCN. Nat med 6: 529–535 [DOI] [PubMed] [Google Scholar]
- Tewari M, Dixit VM (1995) Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem 270: 3255–3260 [DOI] [PubMed] [Google Scholar]
- Warmuth M, Damoiseaux R, Liu Y, Fabbro D, Gray N (2003) SRC family kinases: potential targets for the treatment of human cancer and leukemia. Curr Pharm Des 9: 2043–2059 [DOI] [PubMed] [Google Scholar]
- Watt W, Koeplinger KA, Mildner AM, Heinrikson RL, Tomasselli A, Watenpaugh KD (1999) The atomic-resolution structure of human caspase-8, a key activator of apoptosis. Structure 7: 1135–1143 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhu H, Teraishi F, Davis JJ, Guo W, Fan Z, Fang B (2005) Accelerated degradation of caspase-8 protein correlates with TRAIL resistance in DLD1 human colon cancer cell line. Neoplasia 7: 594–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Salvesen GS (1997) Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem 272: 7797–7800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


