Abstract
Background
We have recently reported that spontaneous internal desynchronization between the locomotor activity rhythm and the melatonin rhythm may occur in rats (30% of tested animals) when they are maintained in constant dim red light (LLdim) for 60 days. Previous work has also shown that melatonin plays an important role in the modulation of the circadian rhythms of running wheel activity (Rw) and body temperature (Tb). The aim of the present study was to investigate the effect that desynchronization of the melatonin rhythm may have on the coupling and expression of circadian rhythms in Rw and Tb.
Methods
Rats were maintained in a temperature controlled (23–24°C) ventilated lightproof room under LLdim (red dim light 1 μW/cm2 [5 Lux], lower wavelength cutoff at 640 nm). Animals were individually housed in cages equipped with a running wheel and a magnetic sensor system to detect wheel rotation; Tb was monitored by telemetry. Tb and Rw data were recorded in 5-min bins and saved on disk. For each animal, we determined the mesor and the amplitude of the Rw and Tb rhythm using waveform analysis on 7-day segments of the data. After sixty days of LLdim exposure, blood samples (80–100 μM) were collected every 4 hours over a 24-hrs period from the tail artery, and serum melatonin levels were measured by radioimmunoassay.
Results
Twenty-one animals showed clear circadian rhythms Rw and Tb, whereas one animal was arrhythmic. Rw and Tb rhythms were always strictly associated and we did not observe desynchronization between these two rhythms. Plasma melatonin levels showed marked variations among individuals in the peak levels and in the night-to-day ratio. In six rats, the night-to-day ratio was less than 2, whereas in the rat that showed arrhythmicity in Rw and Tb melatonin levels were high and rhythmic with a large night-to-day ratio. In seven animals, serum melatonin levels peaked during the subjective day (from CT0 to CT8), thus suggesting that in these animals the circadian rhythm of serum melatonin desynchronized from the circadian rhythms of Rw and Tb. No significant correlation was observed between the amplitude (or the levels) of the melatonin profile and the amplitude and mesor of the Rw and Tb rhythms.
Conclusion
Our data indicate that the free-running periods (τ) and the amplitude of Rw and Tb were not different between desynchronized and non-desynchronized rats, thus suggesting that the circadian rhythm of serum melatonin plays a marginal role in the regulation of the Rw and Tb rhythms. The present study also supports the notion that in the rat the circadian rhythms of locomotor activity and body temperature are controlled by a single circadian pacemaker.
Introduction
Circadian rhythms in physiology and behavior have been described in a wide variety of organisms ranging from bacteria to humans. These rhythms are driven by circadian pacemakers that are capable of generating oscillations with a periodicity close to 24 hours. Several studies have shown that in many organisms the rhythms of locomotor activity and body temperature are under circadian control, and, although the level of activity may influence the body temperature, the circadian rhythm of body temperature is not a mere consequence of the circadian rhythm of locomotor activity (Review in [1]).
In mammals, the principal circadian pacemaker is located in the suprachiasmatic nuclei (SCN), bilateral clusters of neurons in the anterior hypothalamus. This circadian pacemaker regulates the different rhythms present in the body in order that the different circadian rhythms remain synchronized and maintain a stable phase relationships among themselves [2]. However, it must be noted that desynchronization among circadian rhythms may occur under specific experimental conditions. For example, spontaneous internal desynchronization between the body temperature (Tb) and locomotor activity rhythms has been observed in reptiles [3] and in the squirrel monkey [4]. In the owl monkey, internal desynchronization between circadian activity and the feeding patterns has also been reported [5]. A recent investigation has shown that exposure to dim illumination may uncouple several circadian rhythms (e.g., sleep, body temperature, locomotor activity and drinking) in the rat [6]. Internal desynchronization has been also reported in humans [7,8], and it is believed to be the cause of several pathologies [9,10].
Previous studies have shown that melatonin is an important component of the mammalian circadian timing system. Exogenous administration of melatonin can entrain the circadian locomotor activity [11-13], and Tb is affected by melatonin levels [12,14]. We have recently reported that desynchronization of the running wheel activity (Rw) rhythm from serum melatonin may occur in rats exposed to constant dim red light (LLdim, [15]). The aim of the present study was to further expand this finding by investigating the effects that such a desynchronization may produce on the coupling and the expression of circadian rhythms of Rw and Tb.
Materials and methods
Twenty-two male Wistar rats (Charles River Laboratory, Wilmington, MA), eight weeks old at the start of experiment, were used in this study. For Tb recording, rats were implanted under anesthesia (ketamine/xylazine, 50 mg/Kg) with a transmitter (XM-FM, Mini-Mitter Inc., Bend, OR). After surgery, animals were immediately returned to their respective cages and allowed to recover for three days. Then, rats were transferred to a temperature controlled (23–24°C) ventilated lightproof room under LLdim (red dim light 1 μW/cm2 [5 Lux]). Light was provided by a special fluorescent fixture (Litho light # 2, lower wavelength cutoff at 640 nm). Rats were individually housed in cages equipped with a running wheel and a magnetic sensor system to detect wheel rotation (Mini-Mitter Inc. Bend, OR). Tb and Rw data were recorded in 5-min bins and saved on disk using specific software (Tau, Mini-Mitters Inc.). For each animal, we determined the mesor and the amplitude of the Rw and Tb rhythm using waveform analysis on 7-day segments of the data.
After 60 days of LLdim exposure, blood samples (80–100 μM) were collected every 4 hours over a 24-hrs period from the tail artery in heparinized tubes. For each animal, the time of sampling was determined based upon each animal's locomotor activity rhythm. CT12 was defined as the time at which an animal began its daily bout of wheel running activity. All other circadian times were calculated relative to CT12. Melatonin was extracted from the serum (50 μM) using chloroform and then melatonin levels were measured by radioimmunoassay using a commercially available kit (ALPCO Diagnostics, Salem, NH). The sensitivity of the assay was 0.2 pg/ml. Intra-Assay variability was 9% and the inter-Assay was 13% (see [15] for more details).
Analysis of the Rw and Tb rhythms were performed on a 7-day segment of the data (i.e., from day 53 to day 60) using the Clock Lab software (Actimetrics, Evanston, IL). All the experiments reported here conformed to the guidelines outlined in the Guide for the Care and Use of Laboratory Animals from the U.S. Department of Health and Human Services and were approved by the Morehouse School of Medicine Institutional Animal Care and Use Committee.
Results
Out of twenty-two animals, twenty-one showed circadian rhythms in Rw and Tb for the entire duration of the experiment, whereas one rat became arrhythmic after 30 days of exposure to LLdim (see Figure 1 and Table 1). No desynchronization between the circadian rhythm of Rw and Tb and no significant changes in the τ of Rw and Tb rhythms were detected during the 60 day period (t-tests, P > 0.1 in all cases, Figure 1).
Figure 1.
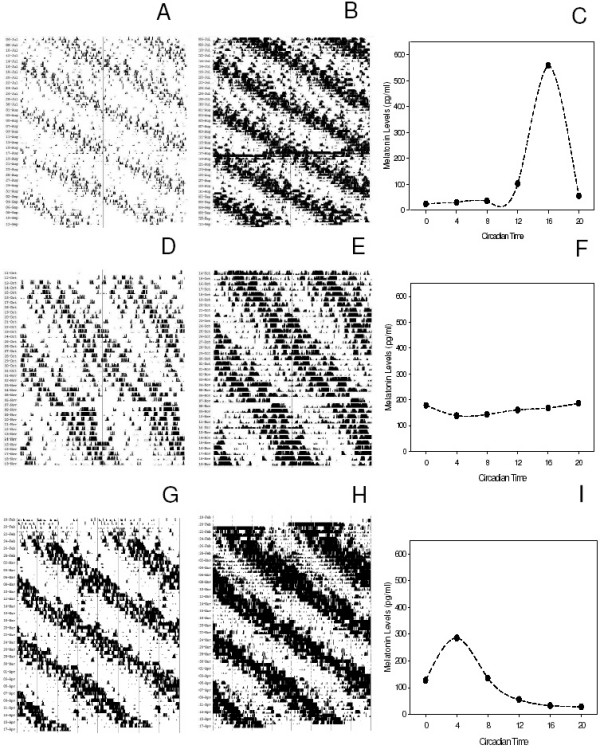
Representative actograms of Rw, Tb and serum melatonin profile. A, B, C: a synchronized animal (rat # 1 in Table 1); D, E. F: an animal with a damped melatonin rhythm (rat # 5 in Table 1); G, H, I: a desynchronized animal (rat # 21 in Table 1). Plots D and E show only the last 40 days of the experiment.
Table 1.
Circadian parameters for Rw and Tb(mean ± SEM) and serum melatonin for each animal tested. Animals in which the circadian rhythm of serum melatonin was desynchronized from Rw and Tb, are indicated in bold. Animals in which the serum melatonin rhythm was damped are indicated in italic. (Amp. = amplitude)
| Running Wheel | Body Temperature | Melatonin | ||||||
| Mesor | Amp. | τ | Mesor | Amp. | τ | Range | Peak | |
| Rat # 1 | 0.6 ± 0.2 | 19.3 ± 3.1 | 25.4 | 37.2 ± 0.1 | 2.1 ± 0.2 | 25.4 | 26–559. | 16 |
| Rat # 2 | 0.7 ± 0.1 | 19.3 ± 3.1 | 25.1 | 36.8 ± 0.1 | 1.8 ± 0.2 | 25.0 | 12–122 | 4 |
| Rat # 3 | 0.1 ± 0.1 | 5.6 ± 1.2 | 24.6 | 37.0 ± 0.1 | 1.9 ± 0.2 | 24.6 | 10–146 | 4 |
| Rat # 4 | NS | NS | 12–544 | NA | ||||
| Rat # 5 | 6.6 ± 1.9 | 45.6 ± 2.1 | 25.1 | 37.6 ± 0.1 | 2.8 ± 0.2 | 25.2 | 105–204 | 16 |
| Rat # 6 | 2.6 ± 0.7 | 39.7 ± 5.0 | 24.3 | 37.5 ± 0.2 | 1.9 ± 0.2 | 24.3 | 38–306 | 16 |
| Rat # 7 | 3.4 ± 1.1 | 30.8 ± 3.1 | 24.4 | 36.9 ± 0.2 | 1.7 ± 0.1 | 24.4 | 109–204 | 20 |
| Rat # 8 | 0.4 ± 0.1 | 8.3 ± 3.4 | 25.0 | 38.5 ± 0.1 | 2.1 ± 0.4 | 25.1 | 32–206 | 20 |
| Rat # 9 | 3.3 ± 0.8 | 48.8 ± 7.7 | 25.3 | 37.3 ± 0.1 | 2.3 ± 0.2 | 25.2 | 138–188 | 20 |
| Rat # 10 | 0.5 ± 0.1 | 12.2 ± 2.0 | 24.3 | 36.8 ± 0.1 | 1.8 ± 0.1 | 24.4 | 108–358 | 4 |
| Rat # 11 | 6.0 ± 1.3 | 53.5 ± 5.2 | 25.1 | 37.6 ± 0.2 | 2.4 ± 0.4 | 25.1 | 128–572 | 0 |
| Rat # 12 | 1.3 ± 0.2 | 23.5 ± 6.1 | 25.2 | 37.4 ± 0.1 | 1.8 ± 0.2 | 25.2 | 158–354 | 16 |
| Rat # 13 | 0.4 ± 0.1 | 8.9 ± 1.1 | 25.1 | 37.1 ± 0.1 | 1.6 ± 0.2 | 25.1 | 97–707 | 20 |
| Rat # 14 | 0.7 ± 0.1 | 15.6 ± 1.3 | 25.2 | 37.3 ± 0.1 | 2.1 ± 0.2 | 25.1 | 144–238 | 20 |
| Rat # 15 | 0.9 ± 0.2 | 15.1 ± 1.4 | 24.6 | 37.5 ± 0.1 | 2.1 ± 0.2 | 24.5 | 118–566 | 8 |
| Rat # 16 | 1.2 ± 0.3 | 24.7 ± 4.0 | 25.1 | 37.6 ± 0.1 | 2.3 ± 0.6 | 25.2 | 155–278 | 16 |
| Rat # 17 | 0.6 ± 0.1 | 14.8 ± 3.1 | 24.3 | 37.2 ± 0.1 | 1.5 ± 0.3 | 24.2 | 26–113 | 8 |
| Rat # 18 | 1.1 ± 0.6 | 15.9 ± 2.8 | 25.0 | 37.4 ± 0.1 | 2.0 ± 0.5 | 25.0 | 20–423 | 16 |
| Rat # 19 | 0.7 ± 0.1 | 15.8 ± 2.5 | 24.5 | 37.7 ± 0.1 | 2.0 ± 0.2 | 24.4 | 20–104 | 16 |
| Rat # 20 | 1.0 ± 0.2 | 16.1 ± 1.3 | 24.5 | 37.6 ± 0.1 | 1.9 ± 0.4 | 24.5 | 21–120 | 16 |
| Rat # 21 | 1.1 ± 0.1 | 20.7 ± 1.9 | 25.4 | 37.4 ± 0.1 | 2.1 ± 0.2 | 25.4 | 27–295 | 4 |
| Rat # 22 | 0.7 ± 0.1 | 13.7 ± 1.5 | 25.1 | 37.4 ± 0.1 | 1.9 ± 0.2 | 25.1 | 34–559 | 20 |
Plasma melatonin levels showed marked variations among individuals in the peak levels and in the night-to-day ratio (Table 1). Interestingly, in six rats the night-to-day ratio was less than 2, whereas in the rat that showed arrhythmicity in Rw and Tb melatonin levels were high and rhythmic with a large night-to-day ratio (Table 1). In seven animals, serum melatonin levels peaked during the subjective day (from CT0 to CT8), thus suggesting that in these animals the circadian rhythm of serum melatonin desynchronized from the circadian rhythms of Rw and Tb (Table 1). No significant correlation was observed between the amplitude (or the levels) of the melatonin profile and the amplitude and mesor of the Rw and Tb rhythms (P > 0.1).
To further investigate the relationships among the Rw, Tb and melatonin rhythms, animals were divided into three different groups: 1) animals (N = 8) in which the rhythm of serum melatonin was synchronized with Rw and Tb rhythms; 2) animals (N = 6) in which the serum melatonin profile was synchronized with the Rw and Tb rhythms but had a reduced (less than 2-fold) amplitude; and 3) animals (N = 7) in which the serum melatonin rhythm was desynchronized from Rw and Tb rhythms.
Figure 1A-C shows representative records of Rw, Tb and melatonin levels obtained in a rat (#1 in Table 1) that did not show desynchronization or a reduced night-to-day serum melatonin ratio. Although melatonin levels in the animals belonging to this group were quite variable, all the animals showed a high night-to-day ratio (Table 1). The mean values of the circadian parameters of Rw and Tb for this group of animals are shown in Table 2.
Table 2.
Circadian parameters for Rw and Tb (mean ± SEM). Group 1 = synchronized animals in which serum melatonin showed a high (more than 5) night-to-day ratio. Group 2 = synchronized animals in which serum melatonin showed a night-to-day ratio smaller than 2. Group 3 = desynchronized animals (i.e., animals in which the serum melatonin levels peaked during the subjective day). No significant differences were observed among the groups in any of the circadian parameters investigated (ANOVA, P > 0.1).
| Running wheel activity | Body Temperature | ||||||
| N | Mesor | Amplitude | τ | Mesor | Amplitude | τ | |
| Group 1 | 8 | 0.9 ± 0.2 | 17.2 ± 3.4 | 24.9 ± 0.1 | 37.5 ± 0.1 | 1.9 ± 0.1 | 24.9 ± 0.1 |
| Group 2 | 6 | 2.7 ± 0.9 | 31.5 ± 5.4 | 25.0 ± 0.1 | 37.3 ± 0.1 | 2.2 ± 0.2 | 25.0 ± 0.1 |
| Group 3 | 7 | 1.4 ± 0.8 | 20.2 ± 5.9 | 24.8 ± 0.2 | 37.2 ± 0.1 | 1.9 ± 0.1 | 24.7 ± 0.2 |
Figure 1D-F shows two representative actograms for Rw and Tb rhythms and the melatonin profile for one animal (# 5 in Table 1) in which the amplitude of the melatonin rhythm was damped (i.e., less than 2 fold). In this group, peak melatonin levels showed less variability (range: 188–354 pg/ml) and the peak levels were lower that those recorded in the previous group (Table 1). Although in these animals the amplitude of the melatonin rhythm was reduced, the free-running period, the amplitude, and the mesor of the RW and Tb rhythms were not different from those observed in the previous group of animals (t-test, P > 0.1 in all cases, Table 2).
Finally, Figure 1G-I shows the records of an animal (# 21) belonging to the group in which desynchronization from the RW and Tb rhythms was observed. Though these animals had a melatonin profile that was desynchronized from the RW and Tb rhythms, melatonin levels showed a marked variation over the 24 h and the peak values were not different from what was observed in the animals that did not desynchronize (t-test, P > 0.5, Table 1). Although in these animals the melatonin rhythms was desynchronized from the RW and Tb rhythms, we did not observe any significant change in τ, amplitude or mesor of the RW and Tb rhythms (t-test, P > 0.1 in all cases, Table 2).
Discussion
The relationship between the circadian rhythms of locomotor activity and body temperature has been investigated in few studies. In humans, it has been reported that the body temperature rhythm is phase-advanced with respect to the activity rhythm [16] and, occasionally, these two rhythms may desynchronize [7,8]. However, studies in other mammalian species failed to observe this phenomenon either in nocturnal or in diurnal mammals [17,18], thus suggesting that in these animals the circadian rhythms of locomotor activity and body temperature are tightly coupled and, most likely, are controlled by a single circadian pacemaker [1,18]. The data obtained in this study support this view because they indicate that the Rw and Tb rhythms in Wistar rats are tightly coupled. Although we did not take special precautions to prevent masking of the Tb rhythm by the Rw rhythm, we observed no desynchronization between the Rw and Tb rhythms.
Our results also indicate that long term exposure to LLdim can induce desynchronization of the circadian rhythm of serum melatonin, and the amplitude of the circadian rhythm in serum melatonin may be dramatically reduced. Moreover, the observation that melatonin remained rhythmic in an animal in which Rw and Tb were arrhythmic further suggests that the regulation of melatonin rhythmicity is independent from the regulation of the running wheel activity and body temperature rhythms. These results confirm and expand our recent study [15] by showing that alteration in some parameters of the melatonin rhythm (i.e., desynchronization and amplitude) had no effects on τ, amplitude and mesor of the Rw and Tb rhythms. Such a result was unexpected because it is believed that melatonin plays an important role in the regulation of the circadian timing system as well as of body temperature [12,14].
Recent experimental evidence suggests that the SCN may contain several circadian pacemakers. For example, the circadian rhythm of arginine vasopressin and vasoactive intestinal polypeptide release in cultured SCN is regulated by different populations that can desynchronize from each other [19,20]. Spontaneous splitting of the locomotor activity rhythm under constant bright light may be the consequence of desynchronization of populations between the left and right SCN [21]. A very recent study using a forced desynchronization protocol has indicated the presence of two oscillators in the anatomically SCN subdivisions [22], thus suggesting that the SCN is composed of different populations of circadian oscillators that constitute regional pacemakers controlling specific circadian outputs.
In mammals the pineal gland is the major source of circulating melatonin, and several studies have shown that melatonin synthesis is under the control of a circadian pacemaker located in the SCN via a multisynaptic pathway [23]. Our study suggests that the circadian pacemaker driving melatonin synthesis is rather independent from the circadian pacemaker(s) driving the locomotor activity and the body temperature rhythms since it can desynchronize or damp without affecting these rhythms and, at the same time, it can remain rhythmic even in the case when Rw and Tb rhythms may became arrhythmic.
Remarkably, the reduced amplitude of the melatonin rhythm observed in several animals (Table 1 and Figure 1F) was caused by a clear increase of the basal melatonin levels and a decrease of peak levels. Such a result is well in agreement with our previous study in which we reported that pineal Arylalkylamine N-acetyltransferase mRNA levels are reduced in animal exposed to LLdim [15] and suggests that, in some animals, the signal by which the SCN drives the circadian rhythm of pineal melatonin synthesis may be reduced under long-term exposure to constant conditions. However, it must be also mentioned that this reduction in the amplitude of the serum melatonin rhythms may be due to the fact that peak and trough levels were missed due to the limited number of sampling points used.
In conclusion, the data presented in this study support the idea that the mammalian SCN is composed of a network of circadian pacemakers that control specific outputs, so that under specific experimental conditions (i.e., exposure to constant dim light or forced desynchrony protocols) these pacemakers may desynchronize. Our data also support the notion that in the rat the circadian rhythms of locomotor activity and body temperature are controlled by a single pacemaker.
Competing interests
The author(s) declare that they have no competing interest.
Authors' contributions
JA and NMB participated in data collection and data analysis. JA drafted the manuscript. GT directed the study and wrote the final version of the manuscript. All authors read and approved the final version of the article.
Acknowledgments
Acknowledgements
This work was supported by the NASA Cooperative Agreement NCC 9–58 with the National Space Biomedical Research Institute to G.T.
Contributor Information
Jacopo Aguzzi, Email: jaguzzi@cmima.csic.es.
Nicole M Bullock, Email: nicolebullock@gmail.com.
Gianluca Tosini, Email: gtosini@msm.edu.
References
- Refinetti R, Menaker M. The circadian rhythm of body temperature. Physiol Behav. 1992;51:613–637. doi: 10.1016/0031-9384(92)90188-8. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Menaker M. SCN: ringmaster of the circadian circus or conductor of the circadianorchestra? Novartis Found Symp. 2003;253:110–121. [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythm of body temperature in an ectotherm (Iguana iguana) J Biol Rhythms. 1995;10:248–255. doi: 10.1177/074873049501000307. [DOI] [PubMed] [Google Scholar]
- Sulzman FM, Fuller CA, Moore-Ede MC. Environmental synchronizers of squirrel monkey circadian rhythms. J Appl Physiol. 1977;43:795–800. doi: 10.1152/jappl.1977.43.5.795. [DOI] [PubMed] [Google Scholar]
- Erkert HG. Internal desynchronization of the circadian activity and feeding rhythm in an owl monkey (Aotus lemurinus griseimembra): a case study. Chronobiol Int. 2000;17:147–153. doi: 10.1081/CBI-100101039. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sagara M, Inoue S. Continuous exposure to dim illumination uncouples temporal pattern of sleep, body temperature, locomotion and drinking behavior in rat. Neurosci Lett. 2000;279:185–189. doi: 10.1016/S0304-3940(99)00943-X. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Gerecke U, Weaver R. Desynchronization of human circadian rhythms. Jpn J Physiol. 1967;17:450–457. doi: 10.2170/jjphysiol.17.450. [DOI] [PubMed] [Google Scholar]
- Lund R. Personality factors and desynchronization of circadian rhythms. Psychomon Med. 1974;36:224–228. doi: 10.1097/00006842-197405000-00005. [DOI] [PubMed] [Google Scholar]
- Koorengevel KM, Beersma DG, Gordijn MC, den Boer JA, van den Hoofdakker RH. Body temperature and mood variation during forced desynchronization in winter depression: a preliminary report. Biol Psychiatry. 2000;47:355–358. doi: 10.1016/S0006-3223(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Winget CM, DeRosha CW, Markley CL, Holley DC. A review of human physiological and performance changes associated with desynchrony of biological rhythms. Aviat Space Environ Med. 1984;55:1085–1096. [PubMed] [Google Scholar]
- Redman JR, Armstrong SM. Reentrainment of rat circadian activity rhythms: effects of melatonin. J Pineal Res. 1988;5:203–215. doi: 10.1111/j.1600-079x.1988.tb00782.x. [DOI] [PubMed] [Google Scholar]
- Slotten HA, Pitrosky B, Pevet P. Influence of the mode of daily melatonin administration on entrainment of rat circadian rhythms. J Biol Rhythms. 1999;14:347–353. doi: 10.1177/074873099129000759. [DOI] [PubMed] [Google Scholar]
- Warren WS, Hodges DB, Cassone VM. Pinealectomized rats entrain and phase-shift to melatonin injections in a dose-dependent manner. J Biol Rhythms. 1993;8:233–245. doi: 10.1177/074873049300800306. [DOI] [PubMed] [Google Scholar]
- Lin MT, Chuang JI. Melatonin potentiates 5-HT(1A) receptor activation in rat hypothalamus and results in hypothermia. J Pineal Res. 2002;33:14–19. doi: 10.1034/j.1600-079X.2002.01867.x. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Aguzzi J, Bullock MN, Tosini G. Effect of long-term exposure to constant dim light on the circadian system of rats. Neurosignals. 2005;14:117–125. doi: 10.1159/000086294. [DOI] [PubMed] [Google Scholar]
- Wever R. Phase-shifts of human circadian rhythms due to shifts of artificial zeitgebers. Chronobiologia. 1980;7:303–327. [PubMed] [Google Scholar]
- Refinetti R. Phase relationship of the body temperature and locomotor activity rhythms in free-running and entrained rats. Biol Rhythm Res. 1997;28:19–24. doi: 10.1076/brhm.28.3.5.19.13127. [DOI] [Google Scholar]
- Refinetti R. Relationship between the daily rhythms of locomotor activity and body temperature in eight mammalian species. Am J Physiol. 1999;277:R1493–R1500. doi: 10.1152/ajpregu.1999.277.5.R1493. [DOI] [PubMed] [Google Scholar]
- Nakamura W, Honma S, Shirakawa T, Honma K. Regionalpacemakers composed of multiple oscillator neurons in the rat suprachiasmatic nucleus. Eur J Neurosci. 2001;14:666–674. doi: 10.1046/j.0953-816x.2001.01684.x. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. Two distinct oscillators in the rat suprachiasmatic nucleus in vitro. Proc Natl Acad Sci USA. 1995;92:7396–7400. doi: 10.1073/pnas.92.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Carpio A, Schwartz WJ. Antiphase oscillation in the left and right suprachiasmatic nuclei. Science. 2000;290:799–801. doi: 10.1126/science.290.5492.799. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Cambras T, Schwartz WJ, Diez-Noguera A. Forced desynchronization of dual oscillators within the rat suprachiasmatic nucleus. Current Biol. 2004;14:796–800. doi: 10.1016/j.cub.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus The Mind's Clock. New York, Oxford University Press; 1991. [Google Scholar]


