Abstract
The histopathological hallmark of lentiviral-associated encephalitis is an abundance of infected and activated macrophages. Why a subset of infected hosts develops lentiviral encephalitis and others do not is unknown. Using a CD8+ T-cell depletion model of simian immunodeficiency virus (SIV)-infected rhesus macaques, we examined the relationship between peripheral SIV infection of monocytes/macrophages and the development of encephalitis. At the same time that cerebral spinal fluid viral load increased in macaques that developed encephalitis, we observed that monocyte-derived macrophages from these macaques produced more virus than those from macaques that did not develop encephalitis. However, during the course of infection, the number of blood monocyte-associated SIV DNA copies did not distinguish macaques that developed simian immunodeficiency virus encephalitis from macaques that did not develop encephalitis. Paradoxically, in this model, macaques that developed encephalitis had fewer SIV-infected macrophages in lungs and thymus at postmortem than macaques that did not develop encephalitis. These findings suggest that inherent differences in host monocyte viral production are related to development of encephalitis.
Approximately one of four immunosuppressed acquired immune deficiency syndrome (AIDS) patients develop a neurodegenerative disorder clinically characterized as human immunodeficiency virus (HIV)-associated dementia complex (HIVD).1 HIVD is a clinical syndrome associated with cognitive, motor, and behavioral deficits that are thought to be mediated by diffuse neuronal damage and loss. In the absence of opportunistic infections of the central nervous system (CNS), HIV encephalitis (HIVE) appears to be the pathological substrate for HIVD.2 The pathological hallmarks of HIVE are microglial nodules, multinucleated giant cells, and the presence of abundant activated or HIV-infected macrophages.3 The pathogenesis of neuronal injury is unknown because there is little evidence of convincing neuronal infection. Current hypotheses suggest a myriad of secreted products from infected and activated macrophages that might interact with neurons or activate astrocytes to initiate synaptic damage followed by neuronal death.4–7
The simian immunodeficiency virus (SIV)-infected macaque model shares numerous characteristics with human HIV infection including development of encephalitis in a variable percentage of infected macaques (∼25%). Factor(s) determining whether macaques develop simian immunodeficiency virus encephalitis (SIVE) have not been defined; however, incidence and speed of onset (∼6 to 36 months) vary with primate species and viral strains.8–10 Macrophage-tropic SIV is the predominant virus found in the CNS of macaques with SIVE.11,12 Abundance of macrophage-tropic variants within the host is necessary, but not sufficient, for development of encephalitis.11,12 This suggests that either additional viral determinants or host factors influence the ability of virus to replicate in brain macrophages. Macaques that exhibit rapid disease progression13 or low anti-SIV antibody titer 1 month after infection14 are more likely to develop encephalitis. Development of two macaque models that have rapid disease progression are associated with increased incidence of CNS disease (80 to 90%).15–17 One model uses infection of pigtailed macaques with two viral strains that results in immunosuppression and replication in macrophages.15 The second model uses treatment of rhesus macaques with an anti-CD8 antibody at the time of infection, resulting in decreased control of viral replication because of depletion of CD8+ T cells and natural killer cells.16,17 These models suggest innate host factors are important determinants of encephalitis. However, it is still unclear why activated and infected macrophages are abundant in the CNS of some lentiviral-infected hosts.
Because HIV and SIV can be recovered from the cerebral spinal fluid (CSF) during the acute phase of infection,18–25 it is possible that virus enters the brain and establishes either a chronic active or a latent infection. Most studies suggest that CNS infection is suppressed while the host has an intact immune response. Both HIV DNA26–28 and SIV DNA29 are present at low levels in brain tissue from asymptomatic individuals. This leaves open the possibility that late stage encephalitis may result from activation of latent CNS virus seeded at the time of primary infection or may result from newly trafficked virus entering the brain within infected macrophages.
It has been reported that the incidence and severity of HIVD30–34 and HIVE have decreased since the advent of highly active anti-retroviral therapy.35 Because highly active anti-retroviral therapy is usually not administered during primary infection, a decrease in incidence of HIVE in individuals on highly active anti-retroviral therapy suggests that suppression of plasma viremia decreases the incidence of encephalitis. Furthermore, infected macrophages in the brains of HIV- and SIV-encephalitic individuals are predominantly distributed in perivascular areas suggesting recent entry.36,37 These observations suggest that development of HIVE may be the result of new virus entering the CNS; however, it is unclear whether these recently entered macrophages were infected before entering the brain or became infected on entering the brain. Because disruption of the physical blood brain barrier is a late event, cell-free plasma virus is unlikely to enter the CNS but rather virus probably enters the CNS within monocytic elements (Trojan horse theory).38,39
It has been theorized that development of encephalitis may be because of increased trafficking of HIV-infected monocytes.40,41 Infection of circulating CD4+ T cells has been studied extensively in both HIV and SIV,42–44 but much less is known regarding infection of circulating monocytes. To begin to understand the role of monocyte infection outside of the CNS during the development of SIVE, it is necessary to longitudinally compare infection of circulating monocytes with the systemic parameters of disease progression and the presence or absence of SIVE at necropsy. We sought to determine whether monocyte/macrophage elements from macaques that develop SIVE harbor more productive infection than macaques that do not develop encephalitis. Using the CD8+ T-cell depletion model, we addressed the following questions with the aim to examine the relationship between peripheral SIV infection of macrophages and the development of SIV encephalitis. Do macaques that develop encephalitis have more circulating infected monocytes or do their monocytes produce more virus after differentiation into macrophages? Is robust macrophage infection unique to the brain or present throughout the body?
Materials and Methods
Animals
Rhesus macaques (Macaca mulatta) were housed and maintained according to American Association of Laboratory Animal Care standards. Macaque information is described in Table 1. Ten rhesus macaques were treated with CD8-depleting antibody17 at −3, 0, and 4 days after infection. Depletion of CD8+ T cells was confirmed by flow cytometry. At day 0, macaques were inoculated with SIVDeltaB670 viral swarm by intravenous injection. Macaques were observed daily for clinical signs of anorexia, weight loss, lethargy, or diarrhea. Two of the macaques were humanely sacrificed at 2 and 4 weeks after infection before onset of clinical signs. The eight remaining macaques were euthanized on development of AIDS. A macaque was considered to have AIDS when SIV infection had progressed to the end stage and was nonresponsive to treatment as determined by clinical observations (eg, increased body temperature, sustained weight loss of 20% or greater, anorexia, increased lymph node size and splenomegaly, changes in activity, diarrhea unresponsive to treatment, opportunistic infections, and changes in general condition), peripheral blood analysis (eg, complete blood cell counts/differentials), and T-cell subset changes. Animals moribund with AIDS were euthanized. Ages of the macaques ranged from 22 to 46 months (age at time of necropsy). Complete necropsies were performed after humane sacrifice.
Table 1.
Rhesus Macaque Age, Sex, Infection Parameters, and Neuropathological and Clinical Diagnosis
| Macaque number | Age (months) | Sex | CD8 depletion (wpi) | Disease at time of sacrifice | Length of infection (days)* | Neuropathological diagnosis | Clinical diagnosis | |
|---|---|---|---|---|---|---|---|---|
| SIV encephalitis | M139 | 34 | M | Yes | AIDS | 56 | SIVE, meningitis, SIV myelitis | Open-mouth breathing; anorexia; diarrhea |
| M140 | 46 | M | Yes | AIDS | 56 | SIVE, SIV myelitis | Anorexia, diarrhea | |
| M144 | 39 | M | Yes | AIDS | 101 | SIVE, SIV myelitis | Bloody nose; lethargic; difficulty breathing | |
| SIV without encephalitis | M141 | 22 | M | Yes | AIDS | 69 | NPC | Diarrhea |
| M135 | 34 | M | Yes | AIDS | 80 | Leukoencephalitis,‡ meningitis | Scrotal and facial edema | |
| M158 | 23 | M | Yes | AIDS | 81 | Meningitis | Anorexia, diarrhea | |
| M145 | 45 | M | Yes | AIDS | 192 | NPC | Lymphadenopathy | |
| M147 | 41 | M | Yes | AIDS | 192 | NPC | Lymphadenopathy | |
| SIV without encephalitis | M154 | 32 | M | Yes | Asymptomatic | 16 | Choroid plexitis | Ataxic |
| Timed sacrifice† | M152 | 39 | M | Yes | Asymptomatic | 30 | Meningitis, choroid plexitis | Diarrhea |
| Noninfected controls | M405 | na | M | No | NA | NA | Control | NA |
| M421 | 66 | F | No | NA | NA | Control | NA |
wpi, weeks post-infection; NA, not applicable; na, not available; NPC, no pathological changes.
A macaque is considered to have AIDS when progressing to the end stages of SIV infection as determined by clinical observations, peripheral blood analysis, and T-cell subset changes. See Materials and Methods for detailed description.
The two macaques that were sacrificed before developing AIDS were not included in the statistical analyses or displayed in graphs unless noted.
This macaque had a mild, focal leukoencephalitis that was not positive for SIV.
Cell Counts
Whole peripheral blood samples obtained from SIV-infected macaques at −3, 0, 4, 7, and 14 days after infection and every 2 weeks thereafter were incubated with fluorochrome-conjugated monoclonal antibodies for 30 minutes at 4°C. For CD4+ and CD8+ T-cell count determination, 100 μl of blood was stained with PerCP-conjugated anti-CD4 (clone L200; BD Biosciences Pharmingen, San Diego, CA), fluorescein isothiocy-anate-conjugated anti-CD3 (clone FN18; Biosource, Ca-marillo, CA), and phycoerythrin-conjugated anti-CD8 (clone DK25; DakoCytomation; Carpinteria, CA). For CD4+CD29+ T cells, 100 μl of blood was stained with PerCP-conjugated anti-CD4, fluorescein isothiocyanate-conjugated anti-CD3, and phycoerythrin-conjugated anti-CD29 (clone 4B4LDC9LDH8; Beckman Coulter, Hialeah, FL). For monocyte count determination, 100 μl of blood was stained with fluorescein isothiocyanate-conjugated anti-CD14 (clone RM052; Beckman Coulter). Red blood cells were lysed using 2 ml of Vitalyse (BioE, Inc., St. Paul, MN) for 30 minutes at room temperature. Cell suspensions were centrifuged and washed with phosphate-buffered saline (PBS) containing 4% fetal bovine serum. Cell suspensions were centrifuged and resuspended in PBS containing 1% paraformaldehyde. The percentages of CD8+/CD3+, CD4+/CD3+, CD4+/CD3+/CD29+, and CD14+ cells were determined on an XL2 flow cytometer (Beckman Coulter). Absolute cell numbers were calculated by multiplying the percentage of cells by the absolute lymphocyte or monocyte counts obtained from blood differential cell counts as previously described.45
CD8 Depletion
The cMT-807 mAb was obtained from Dr. Keith A. Reimann through the National Institutes of Health National Center for Research Resources. Ten rhesus macaques were treated with anti-CD8 monoclonal antibody cM-T807 as previously described.17 Briefly, 3 days before inoculation with SIVDeltaB670, each macaque was administered 10 mg/kg of cM-T807 subcutaneously. On days 0 and 4 after infection, each macaque was administered 5 mg/kg of cM-T807 through intravenous injection.
Tissue
Blood samples were obtained immediately before infection and after infection on days 3, 7, 14, 21, and 28 and every 2 weeks thereafter. CSF draws were attempted every 2 weeks after infection. CSF was aliquoted and stored at −80°C. Brains were removed immediately after euthanasia and processed for analysis. With the exception of M141, that died unexpectedly, all macaques were perfused with saline before necropsy. Regional samples were cut from the left hemisphere, snap-frozen, and stored at −80°C. The right hemisphere was fixed in 10% buffered formalin (Fisher Scientific, Pittsburgh, PA). Coronal sections were made, and tissue blocks were paraffin-embedded. Six-μm sections were made for histopathological analysis. Portions of liver, lung, small bowel, thymus, spleen, and spinal cord were removed immediately after euthanasia and fixed in 10% buffered formalin. Sections of each organ were made, and tissue blocks were paraffin-embedded. Six-μm sections were made for histopathological analysis.
Histology
To assess each macaque brain for the presence of SIVE, paraffin sections of brain tissue containing neocortical gray and white matter, caudate, putamen, hippocampus, occipital cortex, and cerebellum were stained with hematoxylin and eosin. SIVE was empirically defined as the presence of microglial nodules, multinucleated giant cells, and profuse perivascular mononuclear infiltrates. The morphological distribution and abundance of macrophage/microglia and SIV-infected cells was assessed using a monoclonal antibody against macrophage/microglia-associated protein CD68 (clone KP1; DakoCytomation) and a polyclonal antibody against the SIV envelope gp110 (generously provides by Dr. Kelly Stefano Cole and Dr. Ron Montelaro, University of Pittsburgh, Pittsburgh, PA), respectively. Three of the ten macaques showed histological findings of SIVE. The remaining seven macaques did not show histopathological features of SIVE. However, some of the macaques showed rare perivascular infiltrates with three of the nonencephalitic macaques showing histological signs of meningitis (Table 1).
Quantitation of SIV RNA in Plasma and CSF
Virions from either 1 ml of plasma or 500 μl of CSF were pelleted by centrifugation at 16,000 × g or 23,586 × g for 1 hour. Total RNA was extracted from the virus pellet using Trizol (Invitrogen, Carlsbad, CA). Real-time reverse transcriptase-polymerase chain reaction (PCR) was performed with 20 μl of each RNA sample as previously described.46 Primers and probes were specific for the SIV U5/LTR region.
SIV DNA Quantitation
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient using lymphocyte separation medium (Mediatech, Inc., Herndon, VA). PBMCs (107) were incubated with CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Magnetic separation was performed using MiniMACS separator with MS columns (Miltenyi Biotec) according to the manufacturer’s recommendations. Purified monocytes were obtained from the positive fraction. Purity was evaluated by incubating a portion of the positive fraction with fluorescein isothiocyanate-conjugated anti-human CD14 (clone RM052; Beckman Coulter) and phycoerythrin-conjugated anti-human CD3 (clone FN18; Biosource) followed by analysis using an EPICS XL-2 flow cytometer (Beckman Coulter). Purity ranged from 95 to 98%. Cells were pelleted at 14,000 rpm for 1 minute and frozen. DNA was isolated from thawed samples using Qiagen DNA blood mini kit (Qiagen, Valencia, CA) and resuspended in 50 μl of water. The total amount of DNA was measured using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Quantitation of cell-associated DNA was performed by real-time PCR in a Prism 7700 (Applied Biosystems, Foster City, CA). The PCR reaction was performed in triplicate adding 47 μl of a PCR master mix containing 5.5 mmol/L MgCl2, 1× PCR buffer A (Applied Biosystems), 300 mmol/L of each dNTP, 400 nmol/L of each primer, and 200 nmol/L of probe to 3 μl of each sample in a 96-well plate. The primers and probe used were described previously.46,47 To generate a standard curve, serial dilutions of DNA containing the SIV target region, ranging from 101 to 106 copies/reaction, were subjected to PCR in triplicate along with experimental samples. SIV DNA copy numbers from unknown experimental samples were calculated from the standard curve. This result was normalized for volume adjustments (no. of SIV DNA copies/cell), multiplied by the number of circulating monocytes/ml blood as determined by complete blood count and differential, and reported as number of SIV DNA copies from CD14+ monocytes/ml blood.
Ex Vivo Cultures to Assess p27 Production
PBMCs were isolated from whole blood by Ficoll-Paque (Amersham Biosciences). For monocyte-derived macrophage (MDM) cultures, 3 × 106 PBMCs were plated in two-well Lab-Tek Permanox chamber slides (Nalge Nunc Int., Rochester, NY) in AIM-V media (Invitrogen) supplemented with 20% fetal calf serum, 10 ng/ml monocyte colony-stimulating factor (Sigma-Aldrich, St. Louis, MO), and 10 ng/ml of granulocyte-monocyte colony-stimulating factor (Sigma-Aldrich). On day 4 of culture, chamber slides were washed three times with sterile PBS to remove nonadherent cells and maintained in AIM-V supplemented with 20% fetal calf serum. Complete media changes were performed at 7, 10, and 14 days after incubation. Virus production was measured on day 14 supernatants using the SIV core antigen enzyme-linked immunosorbent assay kit (Beckman Coulter) according to the manufacturer’s recommendations. The MDMs in the chamber slides were washed three times with PBS and fixed with 4% paraformaldehyde. To assess the purity and infection of MDM cultures, slides were immunofluorescently stained for macrophages as described for formalin-fixed paraffin-embedded tissue.46 This affirmed that the majority of cells in the culture were MDMs.
For nonadherent cell cultures, 1 × 106 PBMCs were added to 12-well plates in RPMI 1640 containing L-glutamine (Invitrogen) supplemented with 10% fetal calf serum, 40 U/ml recombinant human interleukin-2 (IL-2) (Roche Diagnostics Corp., Indianapolis, IN), and 5 μg/ml of phytohemagglutinin-L (Roche Diagnostics Corp.). On day 4, phytohemagglutinin-L was removed by washing the cells in RPMI 1640 containing l-glutamine supplemented with 10% fetal calf serum and 40 U/ml IL-2. Complete media changes were performed at 7, 10, and 14 days after incubation. Cells were maintained at a concentration of 1 × 106 cells/ml. Virus production was measured on day 14 culture supernatants using the SIV core antigen enzyme-linked immunosorbent assay kit (Beckman Coulter) according to the manufacturer’s recommendations.
Immunofluorescent Histochemistry
Paraffin sections containing neocortical gray matter, basal ganglia, and hippocampus were stained for macrophage-associated lysosomal marker CD68, SIV envelope protein SIVgp110, microtubule-associated protein-2 (MAP-2) (SMI 52; Sternberger Monoclonals Inc., Lutherville, MD), synaptophysin (SY 38; DakoCytomation), or glial fibrillary acidic protein (GFAP) (6F2; DakoCytomation) and detected with fluorogen tags as described previously.46 Double-label immunofluorescence stains using antibodies from the same species were performed using tyramide signal amplification (Perkin-Elmer Life and Analytical Sciences, Boston, MA) for one of the labels.48
For triple-label immunofluorescence, double-label immunofluorescence staining without tyramide amplification was performed first, followed by incubation with a directly conjugated fluorescent monoclonal antibody. GFAP mouse monoclonal antibodies were conjugated with Alexa Fluor 555 using Zenon tricolor mouse IgG1 labeling kit (Molecular Probes, Eugene, OR) according to the manufacturer’s recommendations. The double-labeled immunofluorescent slides were incubated with the conjugated antibody for 2 hours at room temperature. Slides were washed in 0.5% Tween-20 buffer followed by PBS and fixed in 4% formaldehyde in PBS for 15 minutes at room temperature. Slides were mounted in gelvatol.49
Double-label immunofluorescence was performed on paraffin sections of liver, lung, small bowel, thymus, spleen, and spinal cord to assess the number of SIV-infected T cells and macrophages. Staining of the organs was performed as described for brain. A polyclonal antibody (DakoCytomation) or monoclonal antibody (CD3–12; Abcam, Cambridge, MA) against CD3 was used to visualize T cells. The following monoclonal antibodies against macrophage markers were used to determine the identity of SIVgp110+ cells that did not co-label with CD68 or CD3: HLA-DR (DK22; DakoCytomation), HAM56 (HAM56; DakoCytomation), and CD163 (Ber-MAC3; DakoCytomation).
Laser Confocal Microscopy Quantification
Quantification of immunofluorescent staining was performed as described previously.46,50 Regions of macaque brains containing neocortical frontal gray matter, basal ganglia (caudate and putamen), and hippocampus were identified on slides immunofluorescently stained with antibodies to MAP-2, CD68, or synaptophysin. The regions of interest were analyzed by laser confocal microscopy (LSM 510; Zeiss, Jena, Germany). The illumination was provided by argon (458, 477, 488, 514 nm, 30 mW) lasers. Each image was scanned along the z axis and the middle sectional plane was found (262,144 pixels per plane; 1 pixel, 0.25 μm2). Digital images were captured and analyzed with LSM 510 3.2 software (Zeiss).
Each brain region from every macaque was randomly scanned by an individual blinded to the status of the macaques in 10 microscopic areas (×40) encompassing 106,100 μm2. Scanning parameters such as laser power, aperture, gain, and photomultiplier tube settings for each wavelength were kept constant for each macaque specimen. The number of pixels (area) and the intensity of staining [mean fluorescent intensity (MFI)] emitted by each signal were enumerated using a constant threshold that minimized signal because of autofluorescence. The MFI was multiplied by the area stained to measure the total staining for each label in the scanned area. The total staining value (MFI * area) enumerated from the average of 10 scanned areas in a brain region represents a measure of the label in that brain region.
SIV-Infected Cell Counting in Organ Tissue
Slides with sections of liver, lung, small bowel, thymus, spleen, and spinal cord were immunofluorescently stained with antibodies to CD68 and SIVgp110 or CD3 and SIVgp110. Digital images were captured using the LSM 510 3.2 software (Zeiss). Each organ from every macaque was randomly scanned by an individual blinded to the status of the macaques in 10 microscopic areas (×40) encompassing 106,100 μm2. Scanning parameters such as laser power aperture, gain, and photomultiplier tube settings for each wavelength were kept constant for each macaque specimen. Three blinded reviewers enumerated the number of double-labeled cells (CD68+SIVgp110+ or CD3+SIVgp110+) and single-labeled SIV+ cells. The three values from each observer were averaged to represent the number of infected cells in that organ area.
Statistical Analyses
Data were analyzed using Prism 4.0b software (GraphPad Software, Inc., San Diego, CA). We compared each separate variable in two independent, unpaired groups using two-tailed Mann-Whitney tests for nonparametric independent comparisons with 95% confidence intervals. Data were analyzed comparing macaques with SIVE to macaques without encephalitis at each time point rather than comparing the longitudinal trend within the same group. Because two macaques were experimentally sacrificed before developing symptoms that required humane sacrifice, they were not included in statistical analyses comparing macaques with SIVE to macaques without encephalitis.
Results
CD8 Depletion of SIV-Infected Macaques Led to Rapid Progression and SIVE in Three Macaques
Table 1 summarizes clinical and pathological data from 10 SIV-infected and two noninfected rhesus macaques followed in this study. Two of the ten SIV-infected macaques were sacrificed at predetermined time points while they were asymptomatic. These macaques were not included in the statistical analyses, and data were displayed only where noted. The brainstem of one of these macaques (M154) had been accidentally nicked by a needle during a CSF draw. Interestingly, based on immunohistochemical staining, the numerous infiltrating macrophages were not infected with SIV. Inflammation was observed in the choroid plexus of each of the time-sacrificed macaques at necropsy. In M152, there were SIV-infected macrophages in the inflamed choroid plexus. The remaining eight macaques were euthanized on development of clinical AIDS. Three of the eight (38%) macaques developed SIVE (Figure 1). Three other macaques developed meningitis without encephalitis. One macaque (M135) had a mild, focal leukoencephalitis, but this was not classified as SIVE because there were no SIV-infected cells present on immunohistochemical evaluation.
Figure 1.
Triple-label immunofluorescent staining for macrophage marker CD68 (green, Alexa 488), SIV envelope protein (red, Cy5), and astrocyte marker GFAP (blue, Alexa Fluor 555) shows infected macrophages in a microglial nodule from a CD8+ T-cell-depleted rhesus macaque with SIVE (macaque M139). Yellow indicates co-localization of CD68 and SIV. Aqua would show co-localization of SIV and GFAP, however no productively infected astrocytes were detected. Scale bar, 20 μm.
As expected with CD8 depletion, survival time after infection was short for all macaques that developed AIDS (range, 56 to 192 days; mean, 103.4 days; median, 80.5 days). If rapid progression is defined as death from AIDS within 200 days of infection,13 then all eight CD8-depleted macaques were rapid progressors. The average survival time for macaques that developed SIVE (mean, 71 days; median, 56 days) was shorter than macaques that did not develop SIVE (mean, 122.8 days; median, 81 days), but this was not statistically significant (P = 0.48).
Monoclonal antibody against CD8 administered around the time of infection effectively reduced circulating CD8+ T cells to ∼97% (0 to 14 days after infection) and ∼85% (21 to 28 days after infection) of preinfection levels (Figure 2b). CD8+/CD3− cells were also depleted during this time period (data not shown). Surprisingly, macaques that developed SIVE regained circulating CD8+ T cells sooner than macaques that did not develop encephalitis at 2 weeks after infection (P < 0.05), although they never reached preinfection levels during the course of infection (Figure 2b). Previous studies treating macaques with the same CD8-depleting antibody or an irrelevant antibody showed preservation of function in other arms of the immune system.16,17 General immune activation was not observed in noninfected macaques treated with the CD8-depleting antibody. As noted in these previous studies, histological evaluation of lymph node biopsies did not demonstrate evidence of pathological changes associated with antibody treatment.
Figure 2.
Longitudinal peripheral blood counts for CD4+ lymphocytes (a), CD8+ lymphocytes (b), and monocytes (c) of eight CD8-depleted rhesus macaques infected with SIV/DeltaB670. Based on histological findings, macaques were retrospectively classified at postmortem for presence of SIV encephalitis. CD4+ and CD8+ lymphocyte profiles of animals that did or did not develop encephalitis were similar; however, peripheral blood CD4+CD29+ T-helper lymphocytes (d) decreased at 2 weeks after infection in macaques that developed encephalitis. Macaques that developed encephalitis had lower average white blood cell counts before infection or CD8 depletion. a: Peripheral blood absolute CD4+ lymphocyte counts decreased throughout the course of infection. Median peripheral blood absolute CD4+ lymphocyte counts for the three macaques with SIVE are shown in black and the five macaques without encephalitis are shown in gray. b: Peripheral blood CD8+ lymphocytes were suppressed for 2 to 4 weeks in macaques administered cM-T807 during primary infection. Median peripheral blood absolute CD8+ lymphocyte counts for the three macaques with SIVE are shown in black and the five macaques without encephalitis are shown in gray. The asterisk at day 14 after infection indicates a statistically significant difference in the peripheral blood absolute CD8+ lymphocyte count between macaques with and without encephalitis. *P < 0.05. All other time points are not statistically different. c: Peripheral blood absolute monocyte counts were not statistically different between macaques with and without encephalitis. Median peripheral blood absolute monocyte counts for the three macaques with SIVE are shown in black and the five macaques without encephalitis are shown in gray. d: Peripheral blood CD4+CD29+ T-helper lymphocytes decreased earlier in macaques that developed SIVE. Median percentage of peripheral blood T-helper lymphocytes (% CD4+CD29+/total % CD4+ cells) for the three macaques with SIVE are shown in black and the five macaques without encephalitis are shown in gray.
Peripheral Blood CD4+CD29+ T-Helper Cells Diminished Earlier in Macaques that Developed SIVE
The average absolute CD4+ T-cell and monocyte counts between macaques with and without encephalitis were similar (Figure 2, a and c). Preinfection average CD4+ T-cell counts were lower in macaques that developed SIVE (Figure 2a), but in this small number of animals this difference was not statistically significant. The same trend was observed with average preinfection CD8+ T-cell counts (Figure 2b). The peripheral CD4+CD29+ T-cell subset declined from days 4 to 14 after infection (mean of 24.4 to 3.3%; median of 22.5 to 0%) in macaques that developed encephalitis, whereas macaques that did not develop encephalitis had a less dramatic decline (mean of 32.8 to 22%; median of 31.3 to 18.7%) (Figure 2d). The difference in mean percentage of CD4+CD29+ T cells between macaques that did and did not develop encephalitis approached statistical significance (P = 0.067) at 2 weeks after infection.
Plasma Viremia Was Greater at 1 and 3 Weeks after Infection in Macaques that Developed SIVE
Plasma viremia in all CD8-depleted macaques was high (Figure 3a). In macaques that developed encephalitis, mean/median plasma viremia was 1.5 orders of magnitude higher at 1 and 3 weeks after infection compared to SIV-infected macaques that did not develop encephalitis (P < 0.05). The two macaques that survived for 192 days (M145 and M147) suppressed plasma viremia at 6 weeks after infection (1 to 2 log drop in plasma viremia), whereas all other macaques exhibited unsuppressed plasma viremia throughout the course of infection.
Figure 3.
SIV RNA of eight CD8-depleted rhesus macaques infected with SIV/DeltaB670. Based on histological findings, macaques were retrospectively classified at necropsy for presence of SIV encephalitis. Plasma viral load (a) in macaques that developed encephalitis was significantly higher at 1 and 3 weeks after infection compared to macaques that did not develop encephalitis. Between 6 and 8 weeks after infection, higher CSF viral load (b) distinguishes macaques that developed encephalitis from macaques that did not develop encephalitis. a: Median longitudinal plasma SIV RNA for the three macaques with SIVE are shown in black and the five macaques without encephalitis are shown in gray. Plasma SIV RNA of macaques that developed encephalitis was significantly higher than macaques without encephalitis at 1 and 3 weeks after infection. *P < 0.05. b: Median longitudinal CSF SIV RNA for the three macaques with SIVE are shown in black and the five macaques without encephalitis are shown in gray. CSF SIV RNA of macaques that developed encephalitis was higher than macaques without encephalitis throughout the length of infection, especially during the end stages of infection. Because of unavailable CSF samples, statistical analyses could not be completed for all time points. c: Median CSF SIV RNA for macaques with SIVE (black) and macaques without encephalitis (gray) in weeks before death. Macaques that develop SIVE have more virus in CSF than macaques that do not develop encephalitis in the weeks leading up to death.
CSF of Macaques that Developed SIVE Contained More SIV RNA Beginning at 6 to 8 Weeks after Infection
CSF viral load was similar in all macaques during the first 4 weeks after infection (Figure 3b). Between 6 and 8 weeks after infection, macaques that developed encephalitis showed higher mean/median CSF SIV RNA copies/ml. By 8 weeks after infection, CSF viral loads in macaques that developed SIVE were two orders of magnitude higher than macaques that did not develop encephalitis. Median CSF SIV RNA viral loads were two orders of magnitude higher in macaques that developed SIVE at the time of death (Figure 3c).
Monocyte-Associated SIV DNA Did Not Distinguish Macaques that Developed SIVE from Those that Did Not Develop Encephalitis
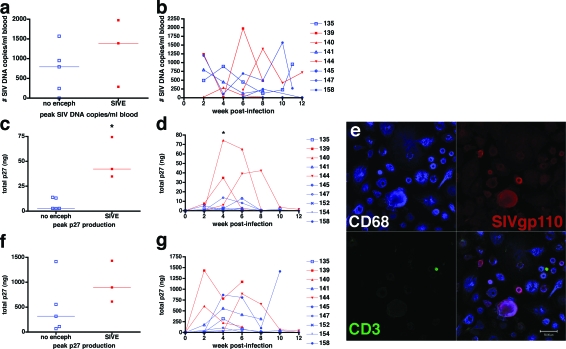
The infection of monocyte/macrophage elements outside of the brain was compared between macaques that developed SIVE and macaques that did not develop encephalitis. The number of SIV DNA copies associated with CD14+ blood monocytes was assessed every 2 weeks after infection in each macaque. For all macaques, the number of SIV DNA copies in monocytes varied from 0 to 1974 SIV DNA copies/ml blood (Figure 4, a and b). One macaque (M145) that did not develop encephalitis did not have any detectable monocyte-associated SIV DNA. Two of the three macaques that developed SIVE (M139 and M144) had higher peak numbers of SIV DNA copies in CD14+ monocytes at 6 and 8 weeks after infection than macaques that did not develop SIVE (Figure 4a); however, one macaque that did not develop encephalitis (M158) had comparable monocyte-associated SIV DNA levels as macaques that developed SIVE at 2 and 10 weeks after infection.
Figure 4.
Analysis of blood monocyte SIV DNA and SIV p27 production in MDMs and nonadherent PBMCs from CD8+ T-cell-depleted rhesus macaques during SIV/DeltaB670 infection. Based on histological findings, macaques were retrospectively classified at necropsy for presence of SIV encephalitis. a: Peak number of SIV DNA copies/ml blood in CD14+ blood monocytes. Each square represents peak number of SIV DNA copies during the course of infection from individual macaques with SIVE (red) and macaques without encephalitis (blue). b: The number of SIV DNA copies was assessed in CD14+ blood monocytes isolated by magnetic bead separation every 2 weeks after infection from macaques with SIVE (red) and macaques without encephalitis (blue). There was a peak in SIV DNA in CD14+ monocytes in two of the three macaques with SIVE from 2 to 8 weeks after infection and one of five macaques without encephalitis at 2 and 10 weeks after infection. Regardless of the development of encephalitis, all but one macaque (no. 145) had detectable SIV DNA in CD14+ blood monocytes at least one time during infection. c: Peak SIV p27 production of MDMs (adherent PBMCs) cultured ex vivo. Each square represents peak p27 production of MDMs during the course of infection from individual macaques with SIVE (red) and macaques without encephalitis (blue). d: During the course of infection, p27 production of MDMs (adherent PBMCs) cultured ex vivo for 14 days showed that the three macaques with SIVE (red) produce more p27 in culture than the five macaques without encephalitis (blue). This graph shows p27 values from MDMs of each macaque every 2 weeks after infection. SIV p27 production peaked in macaques that developed SIVE for one or two consecutive time points measured between 4 to 8 weeks after infection. Average p27 production in MDMs from macaques that developed encephalitis was significantly higher than macaques without encephalitis at 4 weeks after infection, *P < 0.05. Because macaques 152 and 154 were experimentally sacrificed, they were not included in statistical analysis. e: Representative image of MDM cultures that were assayed for SIV p27 production by enzyme-linked immunosorbent assay. The culture was immunostained for CD68 (top left, blue, Cy5), SIVgp110 (top right, red, Cy3), and CD3 (bottom left, green, Alexa 488) and visualized by triple-label immunofluorescent laser confocal microscopy. An overlay of the three preceding images is shown in the bottom right. Purple shows co-localization of MDMs and SIVgp110. f: Peak SIV p27 production of nonadherent PBMCs cultured ex vivo. Each square represents peak p27 production of nonadherent PBMCs during the course of infection from individual macaques with SIVE (red) and macaques without encephalitis (blue). g: Longitudinal p27 production of nonadherent PBMCs cultured ex vivo for 14 days shows that two of three macaques with SIVE (red) produce more p27 in culture than the five macaques without encephalitis (blue) at week 2 after infection. Because of unavailable PBMC samples, statistical analyses could not be completed for week 2 after infection. Because macaques 152 and 154 were experimentally sacrificed, they were not included in statistical analysis. Scale bar, 50 μm.
Ex Vivo SIV p27 Production from Monocyte-Derived Macrophages of Macaques that Developed SIVE Was Higher than Macaques that Did Not Develop Encephalitis at 4 Weeks after Infection
The ability of infected monocytes to replicate virus was assessed ex vivo. Cultured monocyte-derived macrophages were monitored for SIV p27 production every other week after infection to assess viral production in each macaque. Adherent peripheral blood MDMs of macaques that developed encephalitis produced more p27 ex vivo than did MDMs of macaques that did not develop encephalitis (Figure 4, c and d). This difference was observed within 4 to 8 weeks of infection. Peak SIV p27 production of MDMs from macaques that developed SIVE was threefold to fivefold greater than macaques that did not develop SIVE (Figure 4c). To control for potential variability in the number of monocytes plated in each culture, the p27 values were normalized to the number of input monocytes. Normalization showed the same trend and statistical differences (data not shown). Figure 4e shows a representative MDM culture used to assess p27 production. Rare noninfected T cells were occasionally observed; however, all infected cells in these cultures were macrophages. Separate nonadherent PBMC cultures were also monitored for viral production. At 2 weeks after infection, SIV p27 production in nonadherent PBMC cultures (ie, cultures with CD4+ T cells) was higher in two macaques that developed encephalitis than macaques that did not develop encephalitis (Figure 4f); however, after 2 weeks there was no difference in PBMC viral production between macaques that did or did not develop encephalitis (Figure 4g).
At Necropsy, Macaques with SIVE Had More SIV-Infected Cells in Small Bowel and Spinal Cord but Less SIV-Infected Cells in Lung and Thymus than Macaques without Encephalitis
The number of SIV-infected macrophages and SIV-infected T cells in the liver, lung, small bowel, spinal cord, spleen, and thymus at the time of necropsy were compared between macaques with and without encephalitis. Formalin-fixed paraffin-embedded tissue was fluorescently immunostained for macrophages (CD68), T cells (CD3), and virus (SIVgp110). Three observers enumerated the number of infected macrophages (CD68+/SIV+ cells), infected T cells (CD3+/SIV+cells), and SIV-infected cells that did not co-localize with CD68 or CD3 (CD3−/SIV+ and CD68−/SIV+ cells). Figure 5 shows representative fields used to enumerate the number of SIV-infected macrophages and CD3+ T cells in the spinal cord, lung, and thymus (Figure 6). Although many SIV-infected cells in the tissues did not co-label with either CD3 or CD68, of those cells that did double label for SIV and cell-lineage antigens, macrophages were the most common tissue-based infected cell.
Figure 5.
Double-label immunofluorescent staining for macrophage marker CD68 or T-cell marker CD3 and SIV envelope protein shows macaques with SIVE have more infected macrophages in the spinal cord but less infected macrophages in the thymus and lung than macaques without encephalitis. Images such as these were used to count the number of infected macrophages and T cells for tabulation in Figure 6. A–D, F, and G: Small images on the left illustrate individual channels for CD68 (red, Cy5) and SIVgp110 (green, Alexa 488). The large image on the right shows a merged image of the red and green channel with yellow indicating co-localization of CD68 and SIVgp110. E and H: Small images on the left illustrate individual channels for CD3 (red, Cy5) and SIVgp110 (green, Alexa 488). The large image on the right shows a merged image of the red and green channel with yellow indicating co-localization of CD3 and SIVgp110. A: A histological section of spinal cord from a macaque with SIVE (M139). Macrophages are the predominant SIV-infected cell with rare noninfected T cells. B: A histological section of spinal cord from a macaque without encephalitis (M158). Macrophages are less abundant and not infected with SIV. C: A histological section of thymus from a macaque with SIVE (M144). There are few SIV-infected cells and less macrophages than in macaques without encephalitis. D and E: Histological sections of thymus from a macaque without encephalitis (M135). Some of the infected cells in the thymus of macaques without encephalitis were macrophages, fewer were T cells, but the majority of SIV-infected cells did not co-label with either CD68 or CD3. F–H: Histological sections of lung from a macaque without encephalitis (M135) (G and H) and a macaque with SIVE (M139) (F). Some of the infected cells in the lung of macaques without encephalitis were macrophages, but the majority of cells did not co-label with either CD68 or CD3. Macaques with SIVE had few SIV-infected cells in their lungs. Scale bars, 20 μm.
Figure 6.
Necropsy survey of the number of infected macrophages and T lymphocytes observed in peripheral organs from eight CD8-depleted rhesus macaques infected with SIV/DeltaB670. Based on histological findings, macaques were retrospectively classified at postmortem for presence of SIV encephalitis. Each organ was immunostained for both CD3/SIVgp110 and CD68/SIVgp110 and visualized by immunofluorescent confocal microscopy. Three observers enumerated the number of infected macrophages (CD68+/SIV+ cells), infected T cells (CD3+/SIV+cells), and SIV-infected cells that did not co-label with CD68 or CD3 (CD3−/SIV+ and CD68−/SIV+ cells). The black bars represent the mean number of infected cells enumerated for each group (each dot represents the enumeration from an individual field). Macaques with SIVE show more SIV-infected cells in the small bowel and spinal cord but less SIV-infected cells in the lung and thymus than macaques without encephalitis. Many organs have SIV-infected cells that do not co-label with CD3 or CD68. Macrophages are the most common SIV-infected cells in these organs. a: Liver. Median number of infected T cells and macrophages per field in the liver was similar in macaques with and without encephalitis. b: Lung. The median number of infected macrophages and infected cells that did not label with CD68 or CD3 was higher in macaques without encephalitis compared to macaques with SIVE. c: Small bowel. Median number of infected cells that did not label with CD3 or CD68 was statistically significantly higher in macaques with SIVE compared to macaques without encephalitis. d: Spinal cord. Median number of infected macrophages and infected cells that did not label with CD3 or CD68 was statistically significantly higher in macaques with SIVE compared to macaques without encephalitis. e: Spleen. Median number of infected T cells and macrophages per field in the spleen was similar in macaques with and without encephalitis. f: Thymus. The median number of infected macrophages and infected cells that did not label with CD3 was statistically significantly higher in macaques without encephalitis compared to macaques with SIVE. *P < 0.05, **P < 0.01, ***P < 0.001.
SIV-infected cells that did not co-label with CD3 or CD68 had a cytomorphology of macrophages with abundant cytoplasm. In an attempt to identify the origin of these cells, we examined sections of lung from a macaque that had several unidentified SIV-infected cells by staining with macrophage markers HLA-DR, HAM56, and CD163 (data not shown). The SIV-infected cells in the lung were mostly located in the stroma (Figure 5G). Of this array of macrophage markers, CD68 identified the most SIV-infected cells; however, many SIV-infected cells did not co-label with these markers. Not surprisingly, SIV encephalitic macaques had more abundant SIV-infected cells in the spinal cord than macaques without encephalitis (Figure 5, A and B; Figure 6d). However, in other organs (ie, lung and thymus) macaques without encephalitis had more infected cells than encephalitic macaques (Figure 5, C–H; Figure 6, b and f).
The Number of SIV-Infected Cells in Longitudinal Lymph Node Biopsies Did Not Distinguish Macaques that Developed SIVE from Those that Did Not Develop SIVE
The number of SIV-infected macrophages and SIV-infected T cells were also enumerated in lymph node biopsies throughout the course of infection. Two of the three macaques that developed SIVE had more CD68+/SIV+ cells in lymph nodes at 6 weeks after infection than macaques that did not develop encephalitis (data not shown). At 4 weeks after infection, lymph nodes in most macaques had an increase in SIV-infected cells (CD3+/SIV+ and CD3−/CD68−/SIV+ cells) regardless of development of encephalitis. At death, few SIV-infected cells were present in lymph nodes.
Macaques with SIVE Showed More Abundant CD68 Staining in Cortical Regions than Macaques without Encephalitis
All brain regions of macaques with SIVE showed increased CD68 macrophage staining compared to noninfected macaques. The greatest fold increase in CD68 staining was seen in the CA1 and CA4 regions of the hippocampus (∼11,000- and 10,000-fold, respectively) (Figure 7c). Frontal cortical gray and white matter also exhibited 29- and 25-fold increased CD68 staining, respectively, compared to noninfected macaques (P > 0.001). Increased CD68 staining was also significantly higher in the hippocampus and frontal cortical gray matter of macaques with SIVE compared to macaques without encephalitis. Macaques with SIVE showed greater CD68 staining in the basal ganglia compared to noninfected macaques; however, the increase did not achieve statistical significance.
Figure 7.
Analysis of post- and presynaptic proteins and macrophages in the brains of eight CD8-depleted rhesus macaques infected with SIV/DeltaB670 at necropsy. Based on histological findings, macaques were retrospectively classified postmortem as having SIV encephalitis (enc), without encephalitis (no enc), or noninfected controls (con). Each indicated brain region was immunostained for MAP-2 (a), synaptophysin (b), and CD68 (c) and visualized by immunofluorescent confocal microscopy. Ten microscopic fields in each brain region were quantified for each animal and marker. MFI multiplied by the number of pixels (area) covered by fluorescence was quantified in each brain region using immunofluorescent confocal laser microscopy as described in Materials and Methods. For the basal ganglia region, average MFI and pixels for MAP-2 and synaptophysin were compared to macaques without encephalitis and reported as average MFI* area because of limited availability of basal ganglia tissue from noninfected macaques. The black bars represent the median number of infected cells enumerated for each group, whereas each dot represents the enumeration from an individual field. Macaques with SIVE showed less postsynaptic protein (MAP-2 staining) but not presynaptic protein (synaptophysin staining) in cortical regions than macaques without encephalitis. Macaques with SIVE showed more CD68 staining in cortical regions than macaques without encephalitis. a: MAP-2. MAP-2, postsynaptic protein, staining is decreased in the brains of macaques with SIVE. b: Synaptophysin, presynaptic protein, staining is similar in the brains of macaques with and without encephalitis. c: CD68. CD68, a macrophage/microglia-associated protein, staining is significantly increased in the brain regions of macaques with SIVE. * P < 0.05, **P < 0.01, ***P < 0.001.
Macaques with SIVE Showed Less Abundant Postsynaptic Proteins but Not Presynaptic Proteins than Macaques without Encephalitis
To determine whether postsynaptic and presynaptic damage was present in CD8-depleted macaques with and without encephalitis, quantification of postsynaptic protein MAP-2 and presynaptic protein synaptophysin (SYN) was performed in gray matter. Average MAP-2 staining in frontal cortical gray matter and basal ganglia was 44% and 57% lower in macaques with SIVE compared to controls (P < 0.001 and 0.05, respectively) (Figure 7a). Average MAP-2 staining in the CA4 region of the hippocampus and frontal cortical gray matter was 69% and 71% lower in macaques with SIVE than noninfected macaques (P < 0.01 and P < 0.001, respectively) (Figure 7a). Macaques without encephalitis exhibited lower staining for MAP-2 in the CA4 region of the hippocampus and frontal cortical gray matter, but the decrease did not achieve statistical significance. Average presynaptic synaptophysin staining in the CA4 region of the hippocampus, frontal cortical gray matter, and basal ganglia was similar in macaques with and without en-cephalitis and noninfected macaques (Figure 7b). Macaques with SIVE exhibited a significant increase in synaptophysin staining in the CA1 region of the hippocampus compared to noninfected macaques (P > 0.05).
Discussion
Correlates of SIVE
We examined the development of lentiviral encephalitis using a CD8+ T-cell-depleted SIV-infected rhesus macaque model. Some previously published correlates of development of lentiviral encephalitis include: elevated CSF viral loads after acute infection,51 rapid disease progression,13,14 elevated CSF monocyte chemotactic protein (MCP)-1 concentrations,52,53 and low anti-SIV antibody titer at 1 month after infection.14 It has been proposed that CSF viral loads that exceed 106 copies/ml might be a surrogate marker for high viral loads in the brain.54 In this study, we examined the relationship between peripheral SIV infection of monocytes/macrophages and the development of encephalitis with the goal of determining whether macrophage infection is unique to the CNS in animals that develop encephalitis.
Features that Distinguished Macaques that Developed SIVE from Those that Did Not Develop Encephalitis
Our data suggest MDMs from macaques that developed SIVE produced more virus ex vivo at 4 to 8 weeks after infection. Limited volumes of blood are available from macaques at each time point of infection, which necessitates measurement of ex vivo viral production in single cultures. Because the peak viral production of MDMs from the three macaques that developed encephalitis occurred at different time points after infection, a statistical difference was only seen at 4 weeks after infection. Therefore, comparison of peak viral production from ex vivo cultured cells is more informative. However, with limited blood volumes and variability between individual macaques, it will be necessary to follow more macaques during the course of infection to confirm whether MDMs from macaques that develop encephalitis are able to produce more virus than macaques that do not develop SIVE. Because there are several time points in which viral production can be measured from MDM cultures but not nonadherent PBMC cultures containing lymphocytes, we are confident that the monocytes were not infected in culture by virus derived from peripheral blood lymphocytes. Although there was no observable or statistical difference in SIV p27 production in nonadherent PBMC cultures containing CD4+ T cells, SIV production was increased in two of the macaques in the SIVE group at 2 and 6 weeks after infection. This opens the possibility that development of SIVE could be associated with the magnitude of total viral production rather than number of circulating infected monocytes. Differential capability of replicating virus during development of SIVE suggests an inherent difference in the ability of individual host monocytes to become infected and/or to produce virus.
In our study, macaques that developed SIVE had unsuppressed plasma viremia after 6 weeks of infection and significantly higher plasma viremia at 1 and 3 weeks after infection compared to macaques that did not develop SIVE. However, the relationship between high plasma viremia and development of encephalitis is unclear. Some studies have found no correlation between plasma viremia and SIVE,51,55 whereas other studies reported 62% of macaques with elevated anti-genemia had SIVE, when only 9% without elevated antigenemia developed SIVE.8 We do not believe that the increased plasma viremia is due to increased blood monocyte production of virus for a number of reasons. First, although blood monocytes do harbor evidence of multiply spliced mRNA indicating ongoing HIV replication,56 before differentiation into macrophages blood monocytes are not active producers of virus in vitro.57–60 Second, although we see increased viral production from adherent cells (macrophages) of macaques with SIVE, the amount of virus the monocytes are producing cannot account for extremely high plasma viremia. Third, activated CD4+ T cells found in peripheral tissues and tissue macrophages are thought to be the source of plasma viremia.61 In our study, host ability to suppress plasma viral replication 6 weeks after infection was independent of CD8 reconstitution and predictive of longer survival and protection from developing encephalitis. This suggests non-CD8-dependent mechanisms might contribute important control of monocyte viral replication and host survival.
CSF viral load was persistently elevated in macaques that developed encephalitis as previously reported by others.51,55 This highlights a potential difference between the CD8+ T-cell-depleted rapid progression simian model and human disease. In HIV infection, virus can be isolated from the CSF during acute infection62; however, during asymptomatic phases CSF viral load is low. After the development of AIDS, some HIV-infected individuals develop HIVE associated with increased CSF viral load.25,54,63 The persistent elevated CSF viral load and severity of CNS disease observed in CD8+ T-cell-depleted macaques might indicate SIVE develops at early stages of disease rather than at late stages as seen in humans. Interestingly, CSF viral load increased during the same time periods after infection (4 to 8 weeks after infection) as ex vivo production was increased in cultured MDMs in macaques that developed SIVE. Further studies are needed to determine whether this association is indicative of the time period when encephalitis develops.
Although absolute CD4+ T-cell counts were not predictors of encephalitis, the CD4+CD29+ subset of CD4+ T cells declined more rapidly in macaques that developed SIVE compared to macaques that did not develop encephalitis. CD29 (β1 integrin) is part of a heterodimer that binds to vascular cell adhesion molecule-1 (VCAM-1) that is expressed on activated endothelial cells.64 Because expression of CD29 is associated with an activated phenotype,65 macaques that develop encephalitis might selectively lose activated CD4+ T cells. Selective loss of this subset of T cells has been shown to be associated with rapid disease progression, another correlate of SIVE.45,66–68
Factors that Did Not Distinguish Macaques that Developed SIVE from Those that Did Not Develop Encephalitis
In this study, CD8+ T-cell depletion at the time of infection led to encephalitis in 38% of the macaques that progressed to AIDS. This percentage is similar to non-CD8+ T-cell-depleted macaques.8,13 Williams and colleagues69,70 have found a higher incidence of encephalitis in macaques that remain CD8 depleted for longer than 28 days. In our study, CD8+ T cells began to reappear in circulation 2 to 3 weeks after depletion. It would have been expected that macaques that regained CD8+ T cells sooner would suppress viral replication more efficiently and delay disease progression, but surprisingly, the opposite was observed, macaques that developed SIVE regained CD8+ T cells sooner than macaques that did not develop encephalitis. This might be explained by differences in CD8+ T-cell function or possible differences in the re-emergence of natural killer cells depleted by anti-CD8 antibody treatment. Finally, other mechanisms of viral suppression might be important determinants of encephalitis.
As previous reports13,46 have shown no correlation between CD4+ T-cell count dynamics and development of encephalitis, these CD8+ T-cell-depleted rhesus macaques also did not show any relationship between total CD4+ T-cell and monocyte counts and the development of encephalitis. The numbers of SIV DNA copies in CD14+ blood monocytes were not consistently higher in macaques that developed SIVE compared to macaques that did not develop SIVE. Although two of the three macaques had higher monocyte-associated SIV DNA at two time points during infection, one macaque that did not develop SIVE had equivalent monocyte-associated SIV DNA at two other time points. Higher loads of monocyte-associated DNA did not translate to increased viral production from ex vivo MDMs in animals that did not develop SIVE. Williams and colleagues70 observed peak monocyte-associated SIV DNA in CD8+ T-cell-depleted macaques between 7 and 14 days after infection. Although this analysis did not distinguish macaques with and without encephalitis, it is possible assessing monocyte-associated SIV DNA earlier in infection or in subsets of CD14+/CD16+ cells may better predict development of encephalitis. These data suggest host factors (such as APOBEC family members,71,72 mutant MCP-1 alleles,73 and TRIM-5alpha74) may be more important during the development of encephalitis than the number of circulating infected monocytes.
Unexpectedly we did not observe a clear relationship between systemic macrophage infection and CNS infection. We initially hypothesized that infected macrophages in other solid organs would correlate with development of encephalitis as reported previously in two animals.75 Because both lentiviral encephalitis and lentiviral pneumonitis are associated with replication in macrophages,76 it is thought that there might be a connection between development of SIV encephalitis and SIV pneumonia. As with previous reports,77 we examined the cell lineage of infected cells in necropsy tissue. In our small study, the lung, thymus, and lymph nodes at week 6 after infection had greater numbers of SIV-infected macrophages in macaques without encephalitis compared to macaques with SIVE. This suggests that development of encephalitis in this model is not associated with a general increase in the number of infected macrophages throughout the body. Few infected CD3+ T cells were observed in any organ including secondary lymphoid tissue. This may simply reflect severe depletion of CD4+ T cells in tissues at the end stages of disease.78–80
A large number of SIV-infected cells that did not co-label with either T-cell marker (CD3) or macrophage marker (CD68) were observed in all tissues. The lineage of the non-double-labeled cells is unknown. Although we favor the interpretation that these infected cells are unlabeled macrophages or lymphocytes, other tissue cells (eg, astrocytes) have been proposed to be infected.81–88 Within the CNS of these macaques, we have been unable to co-localize SIV antigen with neuroglial markers. It is possible that infected cells might down-regulate cell-lineage proteins, complicating immunodetection. Because many of these infected cells have the morphological appearance of macrophages, an array of antibodies against macrophage proteins was tested to determine whether other markers (HLA-DR, HAM56, and CD163) would better identify the lineage of the SIV-infected cells; however, CD68 remained the best marker. Interestingly, we do not observe this technical difficulty in tissues from SIV-infected pigtailed macaques (unpublished observations). Because infected cells may assume aberrant morphology, other probes will be needed to identify the lineage of these infected cells. Alternatively, although unlikely, cell tropism may expand in CD8-depleted macaques.
By chance, the macaque with an accidental needle nick in the brain stem during a routine CSF draw was time-sacrificed at 16 days after infection. This animal had 1.9 × 108 RNA copies/ml plasma. It is remarkable that this 3-day-old breech in the blood brain barrier did not lead to a robust infection of the infiltrating macrophages. This unplanned experiment implies that some innate immunity is preserved at early stages precluding infection of receptive host cells in the host brain. Although an isolated incident, it hints that development of CNS infection depends on factors other than blood-brain barrier defects, high plasma viremia, and lack of CD8+ T cells.
Presynaptic and postsynaptic damage have been reported during HIVE.89–93 Compared to macaques without encephalitis, macaques with SIVE had significantly lower postsynaptic proteins (MAP-2) in midfrontal cortical gray matter and basal ganglia as we have observed previously.46 Because macaques with encephalitis had short survival times, this suggests that primary postsynaptic damage occurs quickly. Macaques without encephalitis also had decreased MAP-2 staining in hippocampus and frontal cortical gray matter raising the question whether a robust systemic infection may contribute to postsynaptic damage. Staining for presynaptic protein (synaptophysin) was paradoxically increased in the hippocampus and cortical gray matter of macaques with SIVE compared to noninfected macaques. It is possible that presynaptic proteins may increase in SIVE as a temporary response to acute neuronal damage (eg, perineuronal net disturbance with formation of aberrant synapses94).
In this study we focused on the relationship between peripheral SIV infection of monocytes/macrophages and the development of encephalitis. At the same time that CSF viral load increased in macaques that developed encephalitis, we observed that viral replication in MDMs from macaques that eventually developed SIVE produced more virus than macaques that did not develop encephalitis independent of CD4+ and CD8+ T-cell counts. However, the number of blood monocyte-associated SIV DNA copies did not distinguish macaques that developed SIVE from those that did not develop encephalitis. Paradoxically, macaques that did not develop encephalitis had more SIV-infected macrophages in the lungs and thymus than macaques with SIVE. This suggests that there may be inherent differences in the ability of individual host monocytes to become productively infected or produce virus in animals that develop SIVE. Future studies will be needed to elucidate whether monocytes from macaques that develop SIVE have greater susceptibility to be infected, produce virus, or traffic into the brain.
Acknowledgments
We thank Jonette Werley for valuable technical assistance; Dawn L. McClemens-McBride, Premeela Rajakuman, and Afrouz Bazmi for assistance in obtaining clinical data for the macaques; Stephanie Casino and Holly Casamassa for valuable veterinary assistance; Kathleen Morgan for assistance in early surveys of SIV infection in peripheral organs; and Keith Reimann and the National Cell Culture Center for the CD8-specific Ab (cM-T807).
Footnotes
Address reprint requests to Clayton A. Wiley, University of Pittsburgh Medical Center, Department of Pathology, 200 Lothrop St., A506, Pittsburgh, PA 15213. E-mail: claytonwiley@comcast.net.
Supported in part by the National Institutes of Health (grants R01 MH64921, R01 MH071151, and K24 MH01717 to C.A.W. and grant RR-16001 to the National Cell Culture Center for production of CD8-specific Ab (cM-T807).
References
- Davies J, Everall IP, Weich S, McLaughlin J, Scaravilli F, Lantos PL. HIV-associated brain pathology in the United Kingdom: an epidemiological study. AIDS. 1997;11:1145–1150. doi: 10.1097/00002030-199709000-00010. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Achim C. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol. 1994;36:673–676. doi: 10.1002/ana.410360422. [DOI] [PubMed] [Google Scholar]
- Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1991;1:163–175. doi: 10.1111/j.1750-3639.1991.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Nottet H, Bar DR, van Hassel H, Verhoef J, Boven LA. Cellular aspects of HIV-1 infection of macrophages leading to neuronal dysfunction in in vitro models for HIV-1 encephalitis. J Leukoc Biol. 1997;62:107–116. doi: 10.1002/jlb.62.1.107. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Requirement for macrophages in neuronal injury induced by HIV envelope protein gp120. Neuroreport. 1992;3:913–915. doi: 10.1097/00001756-199210000-00023. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Clarke JA, McGrath MS, Moore D, McGuire D. Monokine products as predictors of AIDS dementia. AIDS. 1996;10:1495–1500. doi: 10.1097/00002030-199611000-00006. [DOI] [PubMed] [Google Scholar]
- Giulian D, Yu J, Li X, Tom D, Li J, Wendt E, Lin SN, Schwarcz R, Noonan C. Study of receptor-mediated neurotoxins released by HIV-1-infected mononuclear phagocytes found in human brain. J Neurosci. 1996;16:3139–3153. doi: 10.1523/JNEUROSCI.16-10-03139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin GB, Murphey-Corb M, Roberts ED, Didier PJ, Martin LN. Correlates of SIV encephalitis in rhesus monkeys. J Med Primatol. 1992;21:59–63. [PubMed] [Google Scholar]
- Czub S, Muller JG, Czub M, Muller-Hermelink HK. Impact of various simian immunodeficiency virus variants on induction and nature of neuropathology in macaques. Res Virol. 1996;147:165–170. doi: 10.1016/0923-2516(96)80231-7. [DOI] [PubMed] [Google Scholar]
- Zink MC, Amedee AM, Mankowski JL, Craig L, Didier P, Carter DL, Munoz A, Murphey-Corb M, Clements JE. Pathogenesis of SIV encephalitis. Selection and replication of neurovirulent SIV. Am J Pathol. 1997;151:793–803. [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Stephens EB, Galbreath D, Zhu GW, Li Z, Foresman L, Zhao LJ, Pinson DM, Narayan O. Simian immunodeficiency virus SIVmac chimeric virus whose env gene was derived from SIV-encephalitic brain is macrophage-tropic but not neurovirulent. J Virol. 1995;69:1367–1369. doi: 10.1128/jvi.69.2.1367-1369.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, Flaherty MT, Spelman JP, Hauer DA, Didier PJ, Amedee AM, Murphey-Corb M, Kirstein LM, Munoz A, Clements JE, Zink MC. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J Virol. 1997;71:6055–6060. doi: 10.1128/jvi.71.8.6055-6060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland SV, Halpern E, Lackner AA. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J Neurovirol. 1998;4:260–268. doi: 10.3109/13550289809114527. [DOI] [PubMed] [Google Scholar]
- O’Neil SP, Suwyn C, Anderson DC, Niedziela G, Bradley J, Novembre FJ, Herndon JG, McClure HM. Correlation of acute humoral response with brain virus burden and survival time in pig-tailed macaques infected with the neurovirulent simian immunodeficiency virus SIVsmmFGb. Am J Pathol. 2004;164:1157–1172. doi: 10.1016/S0002-9440(10)63204-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Clements JE. A novel simian immunodeficiency virus model that provides insight into mechanisms of human immunodeficiency virus central nervous system disease. J Neurovirol. 2002;8(Suppl 2):42–48. doi: 10.1080/13550280290101076. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Simon MA, Kuroda MJ, Lifton MA, Ollert MW, Vogel CW, Racz P, Tenner-Racz K, Scallon BJ, Dalesandro M, Ghrayeb J, Rieber EP, Sasseville VG, Reimann KA. A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am J Pathol. 1999;154:1923–1932. doi: 10.1016/S0002-9440(10)65450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Rausch DM, Lendvay J, Sharer LR, Eiden LE. Cognitive and motor impairments associated with SIV infection in rhesus monkeys. Science. 1992;255:1246–1249. doi: 10.1126/science.1546323. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Goudsmit J, Paul DA, Morrison SH, Connor EM, Oleske JM, Holland B. Expression of human immunodeficiency virus in cerebrospinal fluid of children with progressive encephalopathy. Ann Neurol. 1987;21:397–401. doi: 10.1002/ana.410210413. [DOI] [PubMed] [Google Scholar]
- Appleman ME, Marshall DW, Brey RL, Houk RW, Beatty DC, Winn RE, Melcher GP, Wise MG, Sumaya CV, Boswell RN. Cerebrospinal fluid abnormalities in patients without AIDS who are seropositive for the human immunodeficiency virus. J Infect Dis. 1988;158:193–199. doi: 10.1093/infdis/158.1.193. [DOI] [PubMed] [Google Scholar]
- Sonnerborg AB, Ehrnst AC, Bergdahl SK, Pehrson PO, Skoldenberg BR, Strannegard OO. HIV isolation from cerebrospinal fluid in relation to immunological deficiency and neurological symptoms. AIDS. 1988;2:89–93. doi: 10.1097/00002030-198804000-00003. [DOI] [PubMed] [Google Scholar]
- Resnick L, Berger JR, Shapshak P, Tourtelotte WW. Early penetration of the blood-brain-barrier by HIV. Neurology. 1988;38:9–14. doi: 10.1212/wnl.38.1.9. [DOI] [PubMed] [Google Scholar]
- Gisslen M, Fuchs D, Svennerholm B, Hagberg L. Cerebrospinal fluid viral load, intrathecal immunoactivation, and cerebrospinal fluid monocytic cell count in HIV-1 infection. J Acquir Immune Defic Syndr. 1999;21:271–276. doi: 10.1097/00126334-199908010-00003. [DOI] [PubMed] [Google Scholar]
- Krivine A, Force G, Servan J, Cabee A, Rozenberg F, Dighiero L, Marguet F, Lebon P. Measuring HIV-1 RNA and interferon-alpha in the cerebrospinal fluid of AIDS patients: insights into the pathogenesis of AIDS dementia complex. J Neurovirol. 1999;5:500–506. doi: 10.3109/13550289909045379. [DOI] [PubMed] [Google Scholar]
- Cinque P, Vago L, Ceresa D, Mainini F, Terreni MR, Vagani A, Torri W, Bossolasco S, Lazzarin A. Cerebrospinal fluid HIV-1 RNA levels: correlation with HIV encephalitis. AIDS. 1998;12:389–394. doi: 10.1097/00002030-199804000-00007. [DOI] [PubMed] [Google Scholar]
- Donaldson YK, Bell JE, Ironside JW, Brettle RP, Robertson JR, Busuttil A, Simmonds P. Redistribution of HIV outside the lymphoid system with onset of AIDS. Lancet. 1994;343:383–385. doi: 10.1016/s0140-6736(94)91222-x. [DOI] [PubMed] [Google Scholar]
- Gosztonyi G, Artigas J, Lamperth L, Webster HD. Human immunodeficiency virus (HIV) distribution in HIV encephalitis: study of 19 cases with combined use of in situ hybridization and immunocytochemistry. J Neuropathol Exp Neurol. 1994;53:521–534. doi: 10.1097/00005072-199409000-00012. [DOI] [PubMed] [Google Scholar]
- Teo I, Veryard C, Barnes H, An SF, Jones M, Lantos PL, Luthert P, Shaunak S. Circular forms of unintegrated human immunodeficiency virus type 1 DNA and high levels of viral protein expression: association with dementia and multinucleated giant cells in the brains of patients with AIDS. J Virol. 1997;71:2928–2933. doi: 10.1128/jvi.71.4.2928-2933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JE, Babas T, Mankowski JL, Suryanarayana K, Piatak MJ, Tarwater PM, Lifson JD, Zink MC. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis. 2002;186:905–913. doi: 10.1086/343768. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 1999;13:1249–1253. doi: 10.1097/00002030-199907090-00015. [DOI] [PubMed] [Google Scholar]
- Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol. 2001;20:146–155. [PubMed] [Google Scholar]
- Geraci AP, Simpson DM. Neurological manifestations of HIV-1 infection in the HAART era. Compr Ther. 2001;27:232–241. doi: 10.1007/s12019-001-0020-6. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Dore G. Decreasing incidence of CNS AIDS defining events associated with antiretroviral therapy. Neurology. 2000;55:1424. doi: 10.1212/wnl.55.9.1424. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, Becker JT, Cohen B, McArthur JC. HIV-associated neurologic disease incidence changes: multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- Masliah E, DeTeresa RM, Mallory ME, Hansen LA. Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS. 2000;14:69–74. doi: 10.1097/00002030-200001070-00008. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan horse mechanism for the spread of visan virus in monocytes. Virology. 1985;147:231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L, Hurtrel M, Maire MA, Vazeux R, Dormont D, Montagnier L, Hurtrel B. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991;139:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Lane JH, Sasseville VG, Smith MO, Vogel P, Pauley DR, Heyes MP, Lackner AA. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J Neurovirol. 1996;2:423–432. doi: 10.3109/13550289609146909. [DOI] [PubMed] [Google Scholar]
- Levy JA, Mackewicz CE, Barker E. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- Aquaro S, Perno CF, Balestra E, Balzarini J, Cenci A, Francesconi M, Panti S, Serra F, Villani N, Calio R. Inhibition of replication of HIV in primary monocyte/macrophages by different antiviral drugs and comparative efficacy in lymphocytes. J Leukoc Biol. 1997;62:138–143. doi: 10.1002/jlb.62.1.138. [DOI] [PubMed] [Google Scholar]
- Pantaleo G, Fauci AS. Immunopathogenesis of HIV infection. Ann Rev Microbiol. 1996;50:825–854. doi: 10.1146/annurev.micro.50.1.825. [DOI] [PubMed] [Google Scholar]
- Martin LN, Murphey-Corb M, Soike KF, Davison-Fairburn B, Baskin GB. Effects of initiation of 3′-azido,3′-deoxythymidine (zidovudine) treatment at different times after infection of rhesus monkeys with simian immunodeficiency virus. J Infect Dis. 1993;168:825–835. doi: 10.1093/infdis/168.4.825. [DOI] [PubMed] [Google Scholar]
- Bissel SJ, Wang G, Ghosh M, Reinhart TA, Capuano S, III, Stefano Cole K, Murphey-Corb M, Piatak MJ, Lifson JD, Wiley CA. Macrophages relate presynaptic and postsynaptic damage in simian immunodeficiency virus encephalitis. Am J Pathol. 2002;160:927–941. doi: 10.1016/S0002-9440(10)64915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DH, Rajakumar PA, Wilson LA, Trichel AM, Fuller JT, Shipley T, Wu MS, Weis K, Rinaldo CR, Haynes JR, Murphey-Corb M. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J Virol. 2002;76:3309–3317. doi: 10.1128/JVI.76.7.3309-3317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Achim CL, Hamilton RL, Wiley CA, Soontornniyomkij V. Tyramide signal amplification method in multiple-label immunofluorescence confocal microscopy. Methods. 1999;18:459–464. doi: 10.1006/meth.1999.0813. [DOI] [PubMed] [Google Scholar]
- Zeller R, Rogers M. Current Protocols in Molecular Biology. Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. New York: John Wiley and Sons, Inc.; 1994:pp 12.11.11–12.11.18. [Google Scholar]
- Jordan-Sciutto KL, Wang G, Murphey-Corb M, Wiley CA. Cell cycle proteins exhibit altered expression patterns in lentiviral-associated encephalitis. J Neurosci. 2002;22:2185–2195. doi: 10.1523/JNEUROSCI.22-06-02185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak MJ, Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Zink MC, Spelman JP, Robinson RB, Clements JE. SIV infection of macaques—modeling the progression to AIDS dementia. J Neurovirol. 1998;4:249–259. doi: 10.3109/13550289809114526. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, Dailey P, Achim CL. Distribution of brain HIV load in AIDS. Brain Pathol. 1998;8:277–284. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth M, Czub S, Sauer U, Koutsilieri E, Haaft P, Heeney J, Stahl-Hennig C, ter Meulen V, Sopper S. Relationship between viral load in blood, cerebrospinal fluid, brain tissue and isolated microglia with neurological disease in macaques infected with different strains of SIV. J Neurovirol. 2000;6:187–201. doi: 10.3109/13550280009015822. [DOI] [PubMed] [Google Scholar]
- Zhu T, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins JI, Corey L. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76:707–716. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman R, Hassan NF, Walker R, Godfrey B, Cutilli J, Hastings JC, Friedman H, Douglas SD, Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989;170:1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naif HM, Li S, Alali M, Chang J, Mayne C, Sullivan J, Cunningham AL. Definition of the stage of host cell genetic restriction of replication of human immunodeficiency virus type 1 in monocytes and monocyte-derived macrophages by using twins. J Virol. 1999;73:4866–4881. doi: 10.1128/jvi.73.6.4866-4881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EA, Chen IS, Zack JA, Leonard ML, O’Brien WA. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J Clin Invest. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonza S, Maerz A, Deacon N, Meanger J, Mills J, Crowe S. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J Virol. 1996;70:3863–3869. doi: 10.1128/jvi.70.6.3863-3869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. HIV-1 pathogenesis. Nat Med. 2003;9:853–860. doi: 10.1038/nm0703-853. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- McArthur JC, McClernon DR, Cronin MF, Nance-Sproson TE, Saah AJ, St. Clair M, Lanier ER. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- Pietschmann P, Cush JJ, Lipsky PE, Oppenheimer-Marks N. Identification of subsets of human T cells capable of enhanced transendothelial migration. J Immunol. 1992;149:1170–1178. [PubMed] [Google Scholar]
- Lobb RR, Hemler ME. The pathophysiologic role of alpha 4 integrins in vivo. J Clin Invest. 1994;94:1722–1728. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seman AL, Pewen WF, Fresh LF, Martin LN, Murphey-Corb M. The replicative capacity of rhesus macaque peripheral blood mononuclear cells for simian immunodeficiency virus in vitro is predictive of the rate of progression to AIDS in vivo. J Gen Virol. 2000;81:2441–2449. doi: 10.1099/0022-1317-81-10-2441. [DOI] [PubMed] [Google Scholar]
- Martin LN, Soike KF, Murphey-Corb M, Bohm RP, Roberts ED, Kakuk TJ, Thaisrivongs S, Vidmar TJ, Ruwart MJ, Davio SR, Tarpley WG. Effects of U-75875, a peptidomimetic inhibitor of retroviral proteases, on simian immunodeficiency virus infection in rhesus monkeys. Antimicrob Agents Chemother. 1994;38:1277–1283. doi: 10.1128/aac.38.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LN, Murphey-Corb M, Mack P, Baskin GB, Pantaleo G, Vaccarezza M, Fox CH, Fauci AS. Cyclosporin A modulation of early virologic and immunologic events during primary simian immunodeficiency virus infection in rhesus monkeys. J Infect Dis. 1997;176:374–383. doi: 10.1086/514054. [DOI] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Chen D, Konig R, Mariani R, Unutmaz D, Landau NR. APOBEC3B and APOBEC3C are potent inhibitors of simian immunodeficiency virus replication. J Biol Chem. 2004;279:53379–53386. doi: 10.1074/jbc.M408802200. [DOI] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, Kulkarni H, Bamshad MJ, Telles V, Anderson SA, Walter EA, Stephan KT, Deucher M, Mangano A, Bologna R, Ahuja SS, Dolan MJ, Ahuja SK. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci USA. 2002;99:13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Desrosiers RC, Hansen-Moosa A, Mori K, Bouvier DP, King NW, Daniel MD, Ringler DJ. Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am J Pathol. 1991;139:29–35. [PMC free article] [PubMed] [Google Scholar]
- Babas T, Vieler E, Hauer DA, Adams RJ, Tarwater PM, Fox K, Clements JE, Zink MC. Pathogenesis of SIV pneumonia: selective replication of viral genotypes in the lung. Virology. 2001;287:371–381. doi: 10.1006/viro.2001.1043. [DOI] [PubMed] [Google Scholar]
- Borda JT, Alvarez X, Kondova I, Aye P, Simon MA, Desrosiers RC, Lackner AA. Cell tropism of simian immunodeficiency virus in culture is not predictive of in vivo tropism or pathogenesis. Am J Pathol. 2004;165:2111–2122. doi: 10.1016/S0002-9440(10)63261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, Orenstein JM, Kotler DP, Fauci AS. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Rosenberg YJ, Zack PM, White BD, Papermaster SF, Elkins WR, Eddy GA, Lewis MG. Decline in the CD4+ lymphocyte population in the blood of SIV-infected macaques is not reflected in lymph nodes. AIDS Res Hum Retroviruses. 1993;9:639–646. doi: 10.1089/aid.1993.9.639. [DOI] [PubMed] [Google Scholar]
- Seidman R, Peress NS, Nuovo GJ. In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acids in skeletal muscle in patients with myopathy. Mod Pathol. 1994;7:369–375. [PubMed] [Google Scholar]
- Rodriguez ER, Nasim S, Hsia J, Sandin RL, Ferreira A, Hilliard BA, Ross AM, Garrett CT. Cardiac myocytes and dendritic cells harbor human immunodeficiency virus in infected patients with and without cardiac dysfunction: detection by multiplex, nested, polymerase chain reaction in individually microdissected cells from right ventricular endomyocardial biopsy tissue. Am J Cardiol. 1991;68:1511–1520. doi: 10.1016/0002-9149(91)90288-v. [DOI] [PubMed] [Google Scholar]
- Reece JC, Handley AJ, Anstee EJ, Morrison WA, Crowe SM, Cameron PU. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J Exp Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin A, Rosati M, Patenaude DJ, Hatzakis A, Kostrikis LG, Lazanas M, Wyvill KM, Yarchoan R, Pavlakis GN. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2002;99:7015–7020. doi: 10.1073/pnas.102672999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, O’Leary TJ, Baskin GB, Benveniste R, Harris CA, Nara PL, Rhodes RH. Immunohistochemical localization of human and simian immunodeficiency viral antigens in fixed tissue sections. Am J Pathol. 1987;127:199–205. [PMC free article] [PubMed] [Google Scholar]
- Nelson JA, Wiley CA, Reynolds-Kohler C, Reese CE, Margaretten W, Levy JA. Human immunodeficiency virus detected in bowel epithelium from patients with gastrointestinal symptoms. Lancet. 1988;1:259–262. doi: 10.1016/s0140-6736(88)90348-0. [DOI] [PubMed] [Google Scholar]
- Marras D, Bruggeman LA, Gao F, Tanji N, Mansukhani MM, Cara A, Ross MD, Gusella GL, Benson G, D’Agati VD, Hahn BH, Klotman ME, Klotman PE. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med. 2002;8:522–526. doi: 10.1038/nm0502-522. [DOI] [PubMed] [Google Scholar]
- Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, Ho DD. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS. 1992;6:65–70. doi: 10.1097/00002030-199201000-00008. [DOI] [PubMed] [Google Scholar]
- Ketzler S, Weis S, Haug H, Budka H. Loss of neurons in the frontal cortex in AIDS brains. Acta Neuropathol (Berl) 1990;80:92–94. doi: 10.1007/BF00294228. [DOI] [PubMed] [Google Scholar]
- Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R. Neocortical damage during HIV infection. Ann Neurol. 1991;29:651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- Everall IP, Luthert PJ, Lantos PL. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991;337:1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Medina-Flores R, Wang G, Bissel SJ, Murphey-Corb M, Wiley CA. Destruction of extracellular matrix proteoglycans is pervasive in simian retroviral neuroinfection. Neurobiol Dis. 2004;16:604–616. doi: 10.1016/j.nbd.2004.04.011. [DOI] [PubMed] [Google Scholar]