Abstract
Pathological and physiological hypertrophy of the heart is associated with decreased expression of the Kv4.3 transient outward current (Ito) channel. The downregulation of channel mRNA and protein, which may be proarrhythmic, is recapitulated with cultured neonatal rat ventricular myocytes treated with Angiotensin II (Ang II). Here we show that the 4.9 kb 3’ untranslated region (3’ UTR) of the Kv4.3 channel transcript confers Ang II sensitivity to a promoter-reporter construct. In contrast, Kv4.2 and Kv1.5 3’ UTR sequences are insensitive to Ang II. Both Kv4.3 3’ UTR reporter mRNA and activity are decreased in Ang II-treated cardiac myocytes, in accordance with a decrease in mRNA stability. This regulation is mediated by AT1 receptors and abolished by NADPH oxidase inhibitors and dominant negative rac. The Ang II effect is also blocked by expression of superoxide dismutase (SOD), but not catalase, showing that superoxide is required. Dominant negative subunits, enzyme inhibitors and hydrogen peroxide experiments show that the ASK1 (Apoptosis Signal-regulating Kinase 1)-p38 kinase pathway mediates downstream signaling from NADPH oxidase. Mechanical stretch also downregulates Kv4.3 3’ UTR reporter activity and this requires AT1 receptors and NADPH oxidase. Thus, activation of AT1 receptors by Ang II or stretch specifically destabilizes cardiac myocyte Kv4.3 channel mRNA by activating NADPH oxidase. These results link long-term control of cardiac K+ channel gene expression to a physiological reactive oxygen species (ROS) signaling pathway.
Keywords: potassium channel, Angiotensin, stretch, Nadph oxidase, ROS, mRNA stability
Introduction
Cardiac hypertrophy, caused by congestive heart failure, hypertension and pregnancy, is associated with a decrease in the transient outward current (Ito) in ventricular cardiac myocytes1,2. The suppression of ventricular Ito is thought to promote calcium influx and myocyte contraction in the early adaptive phase of hypertrophy, but eventually may induce calcium overloading of the sarcoplasmic reticulum that leads to arrhythmic firing and sudden death3,4. The reduction of Ito is likely related to the downregulation of Kv4.3 mRNA and protein that occurs with congestive heart failure in humans and hypertension in animals5,6. The finding that the latter effect is blocked by Angiotensin Converting Enzyme (ACE) inhibition implicated Angiotensin II (Ang II) in the control of cardiac Kv4.3 gene expression7. Follow-up experiments showed that Ang II acts directly on cultured neonatal rat ventricular myocytes via AT1 receptors to decrease Kv4.3 mRNA and protein8. However, the onset of the effect was more rapid than the turnover of the channel mRNA, and Ang II did not act on the Kv4.3 promoter8. This indirect evidence led to the proposal that Ang II destabilizes Kv4.3 mRNA in cardiac myocytes. In contrast, the α1-adrenergic receptor agonist phenylephrine downregulated Kv4.3 promoter activity8, suggesting that Gq-mediated signaling shared by AT1 receptors and α1-adrenergic receptors might not be involved in this Ang II effect on mRNA stability.
It has been recently demonstrated that AT1 receptors can act via the small G-protein rac to stimulate NADPH oxidase in cardiac myocytes9,10. NADPH oxidase produces superoxide, which serves as a physiological reactive oxygen species (ROS) messenger within the cell. Separate studies have also shown that AT1 receptors, rac and ROS production are activated by mechanical stretch of cardiac myocytes11-13. Thus, we hypothesized that Ang II and stretch can act via the AT1 receptor-NADPH oxidase pathway to regulate cardiac myocyte Kv4.3 mRNA stability. Here we clone the 3’ untranslated region (3’ UTR) of the Kv4.3 mRNA and show that Ang II destabilizes reporter construct mRNA containing this sequence. We then use reporter experiments to implicate NADPH oxidase signaling in the control of cardiac channel mRNA stability by Ang II and mechanical stretch.
Materials and Methods
Cloning of channel 3’ UTR sequences and generation of reporter constructs
The cDNA fragments corresponding to the entire 3’UTR of Kv4.3 were amplified by reverse transcription-polymerase chain reaction (RT PCR) from 2 μg total RNA extracted from mouse heart. The first-stand cDNA was obtained by reverse transcription with oligo dT and random hexamer primer using reverse transcription reactions (20 μl) containing 15 mm (NH4)2SO4, 20 mm Tris-HCl (pH 8.8), 2 mmol/l MgCl2, 0.05% Tween-20, 500 μmol/l dNTPs (Roche), 0.2 mmol/l DTT, 10 μmol/l random hexamer primer (Invitrogen), 1 U DNase I (Roche), 1 U RNasin (Invitrogen), and 200 U Superscript II reverse transcriptase (Invitrogen). Reactions were incubated at 42°C for 50 minutes. The primers for RT-PCR were designed from mouse Kv4.3 coding region and mouse ESTs corresponded to mouse Kv4.3 3’UTR region. After sequences were confirmed, the PCR product was ligated into Zero Blunt TOPO PCR Cloning Kit (Invitrogen). These clones and a commercial cDNA clone including polyadenylation site and polyA tail (BMAP002, ResGen) were mapped and assembled to produce a 4.9 kb Kv4.3 3’ UTR sequence by using unique restriction enzyme cutting sites and then subcloned into the PGL3 promoter vector XbaI/SalI sites. Standard molecular approaches were used to generate the PG4.3 constructs described in Figure 1.
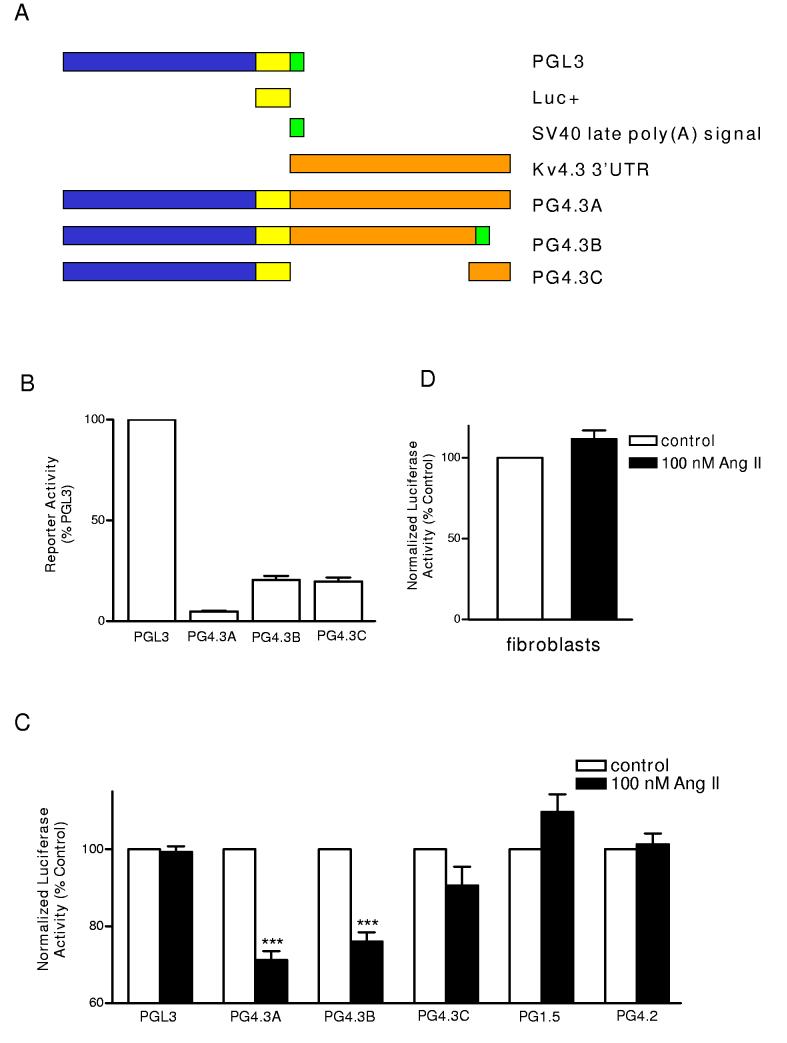
Figure 1.
Effect of Ang II on Kv4.3 3’ UTR reporter constructs. (A) Reporter constructs of various lengths of Kv4.3 3’ UTR. A linearized schematic of the PGL3 vector with luciferase coding sequence (yellow) and the SV40 polyadenylation signal (green) is shown on top. Kv4.3 3’ UTR sequence is indicated by orange. Note that PG4.3B used the PGL3 vector SV40 late polyadenylation site. (B) Basal reporter activity in neonatal ventricular cardiac myocytes. Reporter activity of Kv4.3 3’ UTR constructs are compared to PGL3 in the absence of Ang II (see Materials and Methods for details). (C) Effect of Ang II treatment on reporter activity normalized to cotransfected Renilla luciferase (filled bars). For each construct, we normalized the Ang II data to the normalized activities in vehicle-treated myocytes (open bars). Note that the 3’ UTR constructs derived from the Kv1.5 (PG1.5) and Kv4.2 (PG4.2) were, like PGL3, insensitive to Ang II, while constructs containing most of the Kv4.3 3’ UTR were sensitive to Ang II. n=3-9, *** P< 0.001. (D) Lack of an Ang II effect on PG4.3B expressed in cardiac fibroblasts (n=3).
Similarly, PCR with full length cDNA clones (Clone ID: 4527103 and 30356567, Open Biosystems) was used to introduce 5’ XbaI sites and a 3’ SalI sites into the 0.8 kb Kv1.5 and the 2 kb Kv4.2 3’ UTR sequences. These sequences, including their own polyA tracts, were digested with restriction enzymes, and then 5’ and 3’ fragments were digested with XbaI and SalI, respectively. Finally, the complete 3’ UTR sequences were reassembled and then cloned into the PGL3 vector between the XbaI and SalI sites to generate the 3’ UTR reporter constructs PG1.5 (for the Kv1.5 3’ UTR) and PG4.2 (for the Kv4.2 3’ UTR).
Rat Neonatal Cardiac Myocyte Culture and Treatments
Neonatal rat ventricular cardiac myocytes were isolated from collagenase-treated hearts from 1-day old Sprague-Dawley rats as described previously (8). Following dissociation and preplating for 2 hours to remove nonmyocytes, cardiac myocytes were cultured at low density (0.5 x 106 viable cells per well) in 6 well plates containing in Minimal Essential Medium (MEM) supplemented with 5% calf serum (Gibco) and bromodeoxyuridine (100 μmol/L) to prevent nonmyocyte proliferation. Twenty hours after plating, the cardiac myocytes were transfected using lipofectamine 2000 at a 1:1 ratio for DNA to lipofection reagent for 4 hours with serum-containing medium. Typically, 3 μg of DNA per well was used. However, in experiments comparing various lengths of the Kv4.3 3’ UTR (i.e. Figure 1), molar concentration of reporter plasmid was kept constant (1.5-3 μg of DNA per well was used). Twenty hours after transfection, the medium was replaced with serum-free MEM supplemented with human insulin (10 μg/mL, Sigma), human transferrin (10 μg/mL, Sigma), and bovine serum albumin (1 mg/mL, Sigma). For fibroblast cultures, nonmyocytes isolated in the preplating step were retained and cultured as described above except that bromodeoxyuridine was omitted from the medium.
Two days later, cultures were subjected to treatment with vehicle, 100 nmol/l Ang II or stretch. For experiments utilizing chemical inhibitors, cells were preincubated with inhibitors or vehicle for 30 minutes before treatment (i.e. Ang II or stretch). For dominant negative subunit experiments, transfection with empty vectors was used as a control. For mechanical stretch experiments, myocytes plated on collagen I-coated six well Flexcell culture plates were subjected to a repetitive cyclical stretch paradigm with a 3 second period and 10% average stretch for 6.5 hours using a Flexcell FX4000 strain unit.
Luciferase assay
Renilla luciferase reporter (pRL-TK, Promega) in a 1:10 ratio was cotransfected with each firefly luciferase reporter construct as an internal, non-inducible reporter standard to account for variation in transfection efficiency and global nonspecific effects. Luciferase activity was measured using Dual-Glo luciferase assay system (Promega). Each experiment included three culture wells to generate a single measurement. At least three independent experiments were performed and results are presented with error bars representing the standard error of the mean (SEM).
RNA isolation and Quantitative real-time PCR
Total myocytes RNA was isolated using TRIZOL Reagent (Invitrogen) after transfection and treatment with vehicle or Ang II. DNA was removed by DNase I treatment prior to cDNA synthesis. Relative quantitation by real-time PCR was carried out using SYBR-green detection of PCR products in real time using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems Inc.). In each experiment, the human 18 S RNA was amplified as a reference standard. PCR primers were 18S-F 5’ CCCTGTAATTGGAA-TGAGTCCAC and 18S-R 5’ GCTGGAATTACCGCGGCT; Luc-F 5’ GCCTGAAGTCTCTGATTAAGT and Luc-R 5’ ACACCTGCGTCG-AAGATGT. RT without Superscript II reverse transcriptase and genomic DNA samples were included as negative controls in all real-time PCR experiments. Each real-time PCR reaction (25 μl) contained 2.5 μl of cDNA, (for 18 S RNA detection, cDNAs were diluted 1000-fold), 12.5 μl of 2X SYBR Green Master Mix (Applied Biosystems Inc.) and primers at a final concentration of 350 nmol/l and 900 nmol/l. Reactions were prepared in triplicate and heated to 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 58°C for 60 s, with a final incubation at 95°C for 15 min. To detect the log phase of amplification, the fluorescence quantification of product was determined at each cycle. The cycle at which the fluorescence reached threshold (CT) was recorded, averaged between triplicates and normalized to the averaged CT value for 18 S RNA. The ratio between samples without treatment and with treatment is calculated by Relative quantification14. Results from independent experiments are presented with error bars showing the standard error of the mean (SEM). Melting curves were also measured using Dissociation Curves software (Applied Biosystems) to ensure that only a single product was amplified.
Dominant negative Rac (Rac1 T17N, 3x HA-tagged N-terminus) expression plasmid was obtained from UMR cDNA resource center. Mouse superoxide dismutase (clone ID 5401293) and catalase (clone ID 5050432) expression plasmids were obtained from Open biosystems. Expression plasmids for dominant negative ASK1 (also called HA-ASK1-KM) and dominant negative p38 kinase were kindly provided by H. Ichijo (Tokyo Medical and Dental University) and J. Han (Scripps Research Institute), respectively. Apocynin, diphenylene iodonium and SB 239063 were purchased from Aldrich Chemicals, Sigma-Aldrich Chemicals, and Tocris bioscience, respectively. Candesartan (CV-11974) was kindly provided by AstraZeneca. Controls showing that many of these agents do not act directly on our promoter constructs are shown in the Supplementary material.
Results
The Kv4.3 3’ UTR confers sensitivity to Angiotensin II (Ang II)
We hypothesized that the 3’ untranslated region (3’ UTR) mediates downregulation of cardiac Kv4.3 mRNA by Ang II. Analysis of the Kv4.3 channel coding sequence, promoter and mRNA size8,15 led us to conclude that the 3’ UTR was ∼5 kb in length. We aligned expressed sequence tag (EST) database clones with the genomic mouse Kv4.3 sequence to design primers and then used RT-PCR (reverse transcriptase-polymerase chain reaction) with mouse heart mRNA to clone the first 4868 bases of the 3’ UTR. We then used a commercial EST clone, which includes a polyadenylation site and a polyA tail, to provide the last 29 bases. Sequence analysis showed that the mouse cardiac RT-PCR products are 99% identical to sequences in the mouse EST database (GI # 29356835, 19547440, 24533457, 17962295, 13151612, 3283901 and 50926030), some of which were posted after our cloning.
To test whether the 3’ UTR was responsible for the response to Ang II, we expressed luciferase reporter constructs containing Kv4.3 3’ UTR sequences in neonatal cardiac myocytes (Fig. 1A). The construct of PG4.3A, which contains the complete native Kv4.3 3’ UTR, with a putative polyadenylation site and a polyA tail, produced low expression compared to the PGL3 vector (Fig. 1B). Because this might be due to the noncannonical polyadenylation sequence in the channel construct, we substituted the 3’ 600 bases with the SV40 polyadenylation site from the PGL3 vector to generate the PG4.3B construct (Fig. 1A). This increased expression 5-fold compared to the PG-4.3A construct (Fig. 1B). We then expressed these constructs in neonatal ventricular cardiac myocytes and tested for sensitivity to Ang II. Specifically, we normalized the channel 3’ UTR firefly luciferase reporter activity to a co-transfected constitutively active Renilla luciferase reporter to eliminate variations in transfection and global nonspecific effects. Our measurements showed that treatment with 100 nmol/l Ang II for 7 hours, a period that produces a nearly peak effect on native Kv4.3 channel mRNA8, did not affect normalized reporter expression induced by the control vector (PGL3), a construct containing the 3’ 800 bases of the channel 3’ UTR (PG4.3C), and constructs containing 3’ UTR sequences derived from Kv1.5 (PG1.5) or Kv4.2 (PG4.2). However, Ang II reduced reporter activity in the two constructs that contain the first 4.3 kb of the Kv4.3 3’ UTR (Fig. 1C). Finally, we determined that the Ang II regulation of the Kv4.3 3’ UTR reporter does not occur in cultured neonatal cardiac fibroblasts (Fig. 1D). Thus, the 3’ UTR of the Kv4.3 mRNA specifically confers sensitivity Ang II in cardiac myocytes.
Ang II-induced destabilization of the Kv4.3 3’ UTR
The decrease in reporter activity induced by the channel 3’ UTR could reflect inhibition of luciferase translation or destabilization of the mRNA encoding luciferase. An effect on translation would not affect reporter mRNA concentration, but mRNA destabilization would lower luciferase mRNA levels. In fact, with destabilization the effect on luciferase mRNA should be larger than the change in luciferase activity because luciferase protein must turnover (t1/2∼4 hours, data not shown) to report mRNA downregulation as a decrease in enzyme activity.
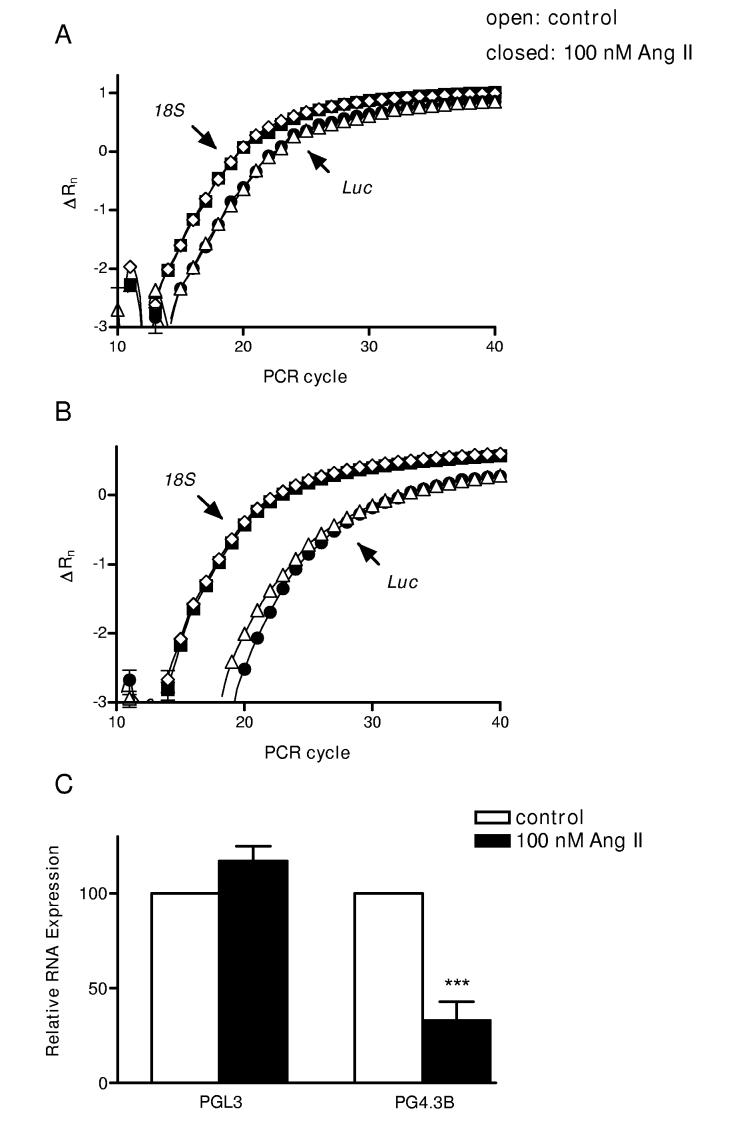
We measured cardiac myocyte cell luciferase transcript levels by real-time PCR. Ang II did not affect the real-time PCR curves (Δ fluorescence versus cycle number) for luciferase mRNA expression induced by the control PGL3 vector (Fig. 2A). Likewise, 18 S ribosomal RNA levels were similar in vehicle and Ang II-treated cells (Fig. 2A,B). However, the PG4.3B curves were right-shifted in Ang II-treated cells (Fig. 2B). In these assays, a rightward shift of one cycle is indicative of a halving in concentration of the reporter mRNA. On average, Kv4.3B luciferase mRNA concentration was reduced 3-fold by Ang II (Fig. 2C). This change is double the effect on luciferase enzyme activity (Fig. 1C) and so is indicative of destabilization of the channel 3’ UTR. Furthermore, because this response is comparable to the effect on native channel mRNA8 (which is too fast to be transcriptional), destabilization of the 3’ UTR fully accounts for Ang II downregulation of Kv4.3 mRNA in neonatal cardiac myocytes.
Figure 2.
Ang II destabilizes the Kv4.3 3’UTR. Representative amplification versus cycle number plots of real-time PCR reactions for Luciferase (△ and ●) and 18 S ribosomal (◇ and ■) RNAs. RNA was isolated from cells transfected with PGL3 vector (A) or the PG4.3B (B). Open symbols show treatment with vehicle for 7 h. Closed symbols show treatment with Ang II. (C) Mean relative expression values from independent RNA isolations from myocytes transfected with PGL3 (left) and the Kv4.3 3’ UTR reporter (PG4.3B) (right). Open bars: vehicle, closed bars: Ang II. n=4, ***p < 0.001.
AT1 Receptor-activated NADPH Oxidase mediates the Ang II-induced mRNA destabilization
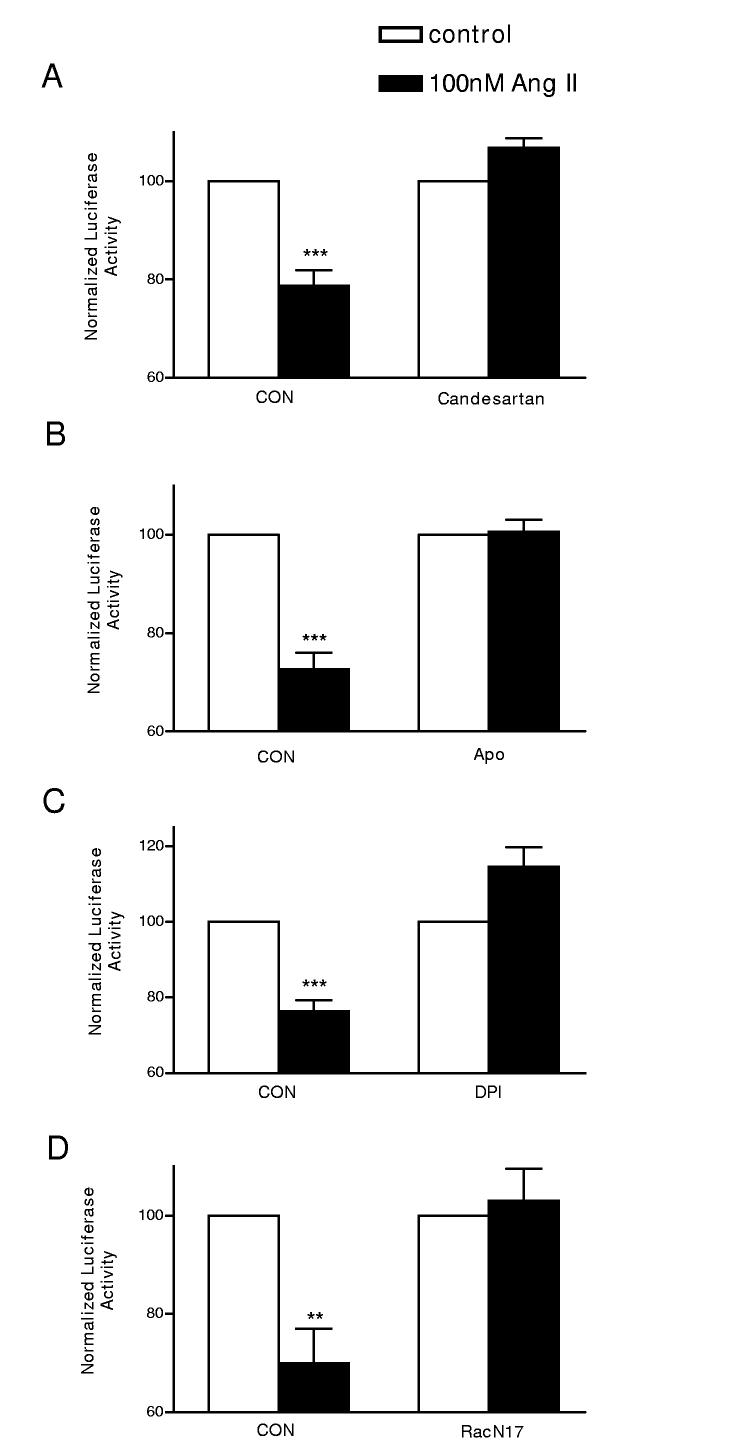
Initial experiments eliminated Ca2+ and protein kinase C as viable messengers in the Ang II effect on endogenous Kv4.3 mRNA (data not shown). Therefore, we began our signaling studies on the PG4.3B reporter by testing whether AT1 receptors mediate the Ang II effect on the 3’ UTR and then examined the role of NADPH oxidase. Figure 3A shows that the Ang II effect was blocked by 100 nmol/l candesartan, a specific AT1 receptor antagonist. In contrast, candesartan did not affect reporter activity induced by the control vector PGL3, PG4.3B or the Kv1.5 3’ UTR reporter construct PG1.5 in the absence of Ang II (Supplemental Fig. S1A-C). Thus, we conclude that the candesartan effect is specific for the Ang II effect on the Kv4.3 3’ UTR. To investigate whether cardiac myocytes utilize NADPH oxidase to downregulate the channel mRNA, we incubated cells with 100 μmol/l Apocynin (Apo) or 10 μmol/l Diphenylene Iodonium (DPI). These structurally and mechanistically distinct inhibitors of NADPH oxidase each abolished the effect of Ang II (Fig. 3B,C). Again, controls with PGL3, PG4.3B and PG1.5 and Apocynin in the absence of Ang II show that this action is specific (Supplemental Fig. S1A-C). Finally, we examined the effect of a dominant negative rac subunit (Rac1 T17N), since the rac G-protein mediates activation of NADPH oxidase by Ang II in cardiac myocytes. As can be seen in Figure 3D, Rac1 T17N also inhibited the Ang II destabilization effect. Hence, NADPH oxidase mediates the AT1 receptor-induced destabilization of the Kv4.3 3’UTR.
Figure 3.
Ang II acts via AT1 receptors, NADPH oxidase and Rac. The effect of 100 nmol/l Ang II on the PG4.3B reporter construct was blocked by 100 nmol/l candesartan, an AT1 receptor antagonist (A), the NADPH oxidase inhibitors 100 μmol/l Apocynin (Apo) (B) and 10 μmol/l diphenylene iodonium (DPI) (C), or expression of Rac1 T17N, a dominant negative Rac subunit. n=3, ***p < 0.001; **p < 0.01.
Downstream signaling from NADPH oxidase
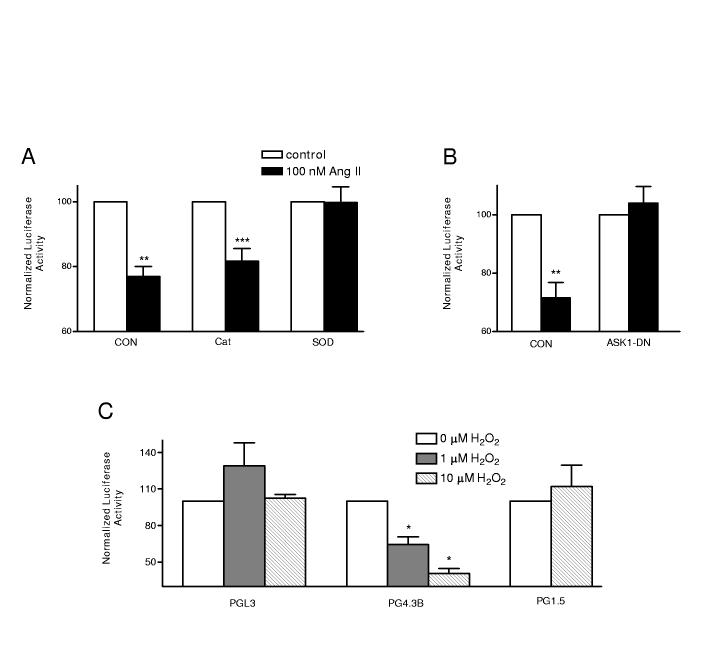
NADPH oxidase generates superoxide that can then be converted into hydrogen peroxide. This conversion is catalyzed by superoxide dismutase (SOD), while hydrogen peroxide is broken down by catalase. Therefore, to test for the involvement of superoxide and hydrogen peroxide in the Ang II effect, cardiac myocytes were transfected with expression vectors for SOD and catalase. If hydrogen peroxide is the physiological mediator, SOD should enhance the response and catalase should inhibit the response. However, we found that catalase left the Ang II effect intact and SOD abolished the Ang II effect on the Kv4.3 3’ UTR (Fig. 4A). SOD did not affect baseline signals from PGL3, PGKv4.3B and PGKv1.5 (Supplemental material Fig. S1). Thus, there were no nonspecific effects or interference with the reporter assay. These results imply that superoxide, not hydrogen peroxide, acts as the physiological mediator of the Ang II effect.
Figure 4.
Superoxide and ASK1 mediate the Ang II effect. (A) Effects of expression of Catalase (Cat) or Superoxide Dismutase (SOD) on Ang II action. (B) Dominant negative ASK1 (ASK1DN) inhibits the Ang II effect. (C) The ASK1 activator hydrogen peroxide mimics Ang II on PG4.3, but does not affect PGL3 or PG1.5. n=3-5, *** p < 0.001, ** p < 0.01, * p<0.05.
Reactive oxygen species (ROS) can activate the Map kinase kinase kinase ASK1 (Apoptosis Signal-regulating Kinase 1)16. To test for involvement of ASK1, cardiac myocytes were transfected with a dominant negative ASK1 (ASK1DN) expression plasmid17. Figure 4B shows that ASK1DN blocks the Ang II effect showing that ASK1 is necessary for this regulation. In contrast, controls were unaffected by ASK1DN (Supplemental material Fig. S1). We then tested whether we could bypass upstream Ang II signaling by using hydrogen peroxide to directly activate ASK116. As can be seen in the PG4.3B results of Figure 4C, bath application of 1 μmol/l hydrogen peroxide mimicked the Ang II response and 10 μmol/l produced a larger effect. In contrast, hydrogen peroxide did not decrease signals from PGL3 and PG1.5 (Fig. 4C). Finally, the effects of hydrogen peroxide and Ang II on the Kv4.3 3’ UTR were not additive (n = 2, Supplemental material Fig. S2A), suggesting that both agents activate the same pathway. Together these results suggest that ASK1 is necessary and sufficient for a specific destabilization of the Kv4.3 3’ UTR.
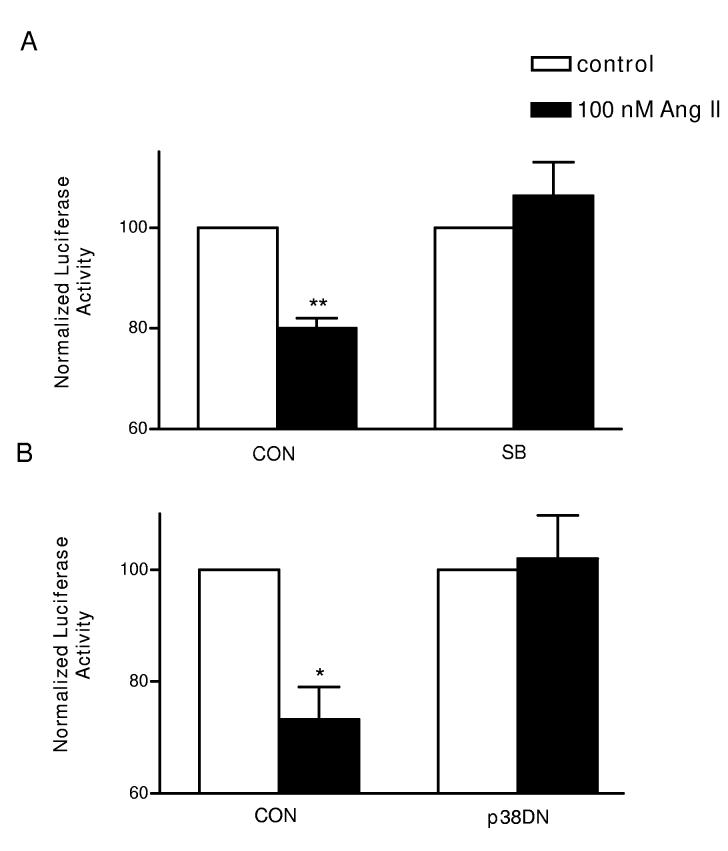
ASK1 is known to induce activation of p38 kinase17, a MAP kinase that is activated in response to cellular stress. Therefore, to test whether p38 kinase is involved in the Ang II-induced destabilization of Kv4.3 mRNA, we first tested for an effect of a chemical inhibitor of p38 kinase on the 3’ UTR reporter. Figure 5A shows that 20 μmol/l SB239063 abolished the Ang II effect. We also found that SB239063 does not affect control constructs (Supplemental material Fig. S1), suggesting that the inhibitor acted specifically. To independently implicate p38 kinase in the Ang II effect, cardiac myocytes were co-transfected with a dominant negative p38 kinase subunit (p38DN)18. Inhibition of p38 by the dominant negative subunit, like SB239063, blocked the Ang II response (Fig. 5B). Therefore, we conclude that the Ang II acts via the ASK1-p38 pathway to destabilize Kv4.3 mRNA.
Figure 5.
p38 is required for the Ang II-induced mRNA destabilization. The Ang II-induced decrease in PG4.3kb reporter activity is blocked by (A) 20 μM SB239063 (SB) or by expression of a dominant negative p38 subunit (p38DN). n=3, ** p < 0.01, * p< 0.05.
Stretch-induced downregulation of cardiac Kv4.3 mRNA
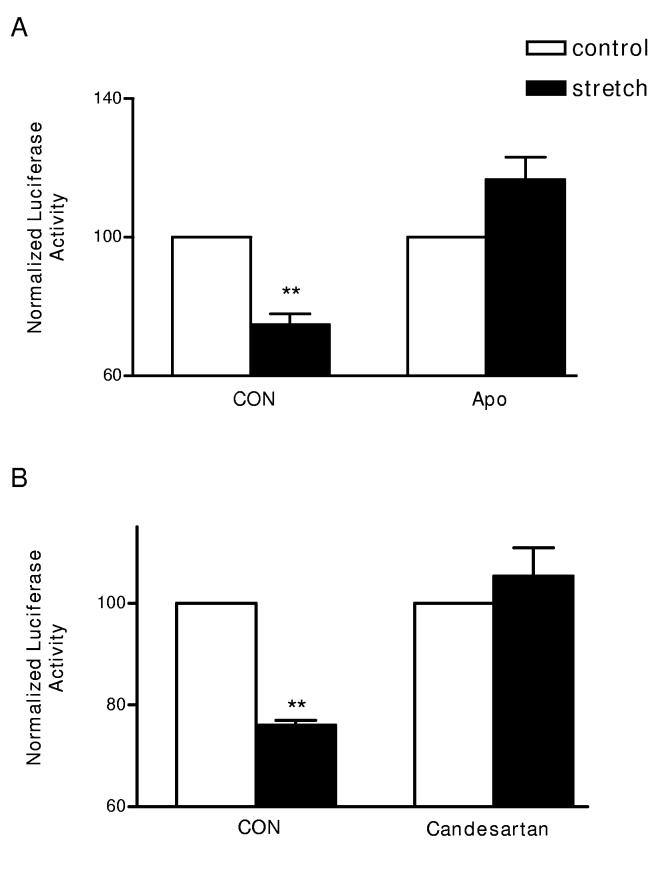
Mechanical stretch of cardiac myocytes is known to activate rac and to induce generation of ROS11,12. Recently, it has also been found that stretch directly activates cardiac myocyte AT1 receptors, even in the absence of Ang II13. Since all of these stretch targets are involved in the signaling described here, we explored whether stretch acts on the Kv4.3 3’ UTR. Cardiac myocytes were plated on collagen I-coated flexible plates and then subjected to repetitive stretch for 6.5 hours. Figure 6A, left shows that stretch decreased 3’ UTR PG4.3B reporter activity. In contrast, mechanical stretch does not affect PGL3 and PG4.2 (n=2, Supplementary material Fig. S2B), showing that this regulation is specific for the Kv4.3 3’ UTR. Furthermore, Figure 6A, right shows that this stretch effect on PGKv4.3B was abolished by the NADPH oxidase inhibitor Apocynin. Likewise, candesartan, a known blocker of stretch activation of AT1 receptors13, abolished the stretch response (Fig. 6B). Given that Apocynin and candesartan do not produce nonspecific effects in controls (Supplementary material, Fig. S1), we conclude that mechanical stretch acts via AT1 receptors and NADPH oxidase to specifically downregulate Kv4.3 mRNA in cardiac myocytes.
Figure 6.
Mechanical stretch acts via AT1 receptors and NADPH oxidase to downregulate Kv4.3B 3’ UTR reporter activity. Cyclical stretch (filled bars) is compared to matched cultures that were not subjected to any stretch (open) bars. (A) Apocynin (Apo) blocks the stretch response. (B) Candesartan inhibits the stretch response. n=3, ** p < 0.01.
Discussion
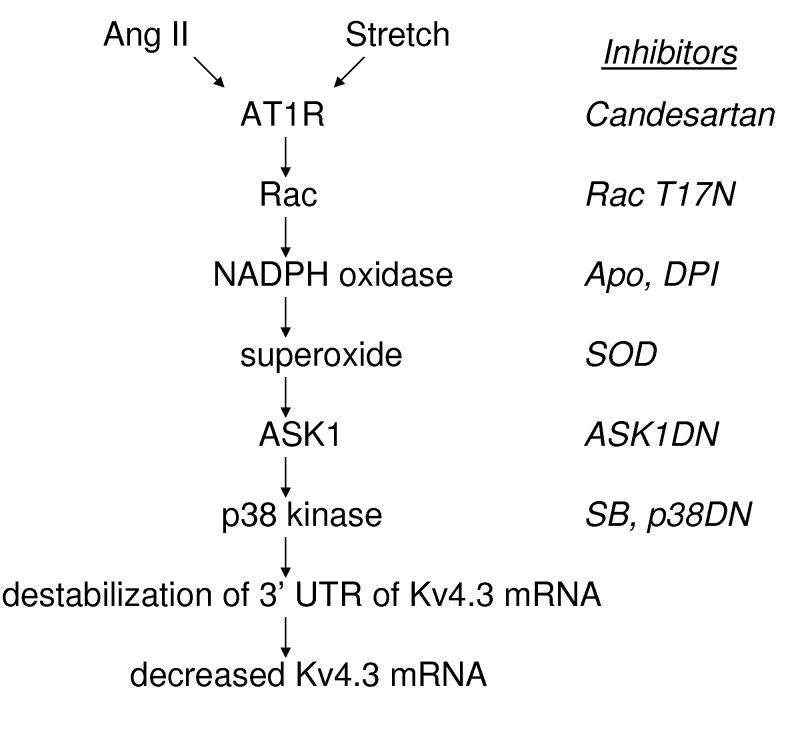
Cardiac hypertrophy is associated with decreased Kv4.3 channel expression, which is thought to be proarrhythmic. In vivo ACE (angiotensin converting enzyme) inhibitor effects led to the demonstration that Ang II can act directly on cardiac myocytes to decrease Kv4.3 mRNA7,8. However, the kinetics of the Ang II effect along with studies of the Kv4.3 promoter raised the possibility that the channel mRNA was destabilized by AT1 receptors. Here studies based on generating reporter constructs with the cloned cardiac mRNA sequences show that Ang II does indeed destabilize the Kv4.3 mRNA and localizes this regulation to the 3’ UTR. Furthermore, we demonstrate that NADPH oxidase generated-superoxide acts via ASK1 and p38 kinase to mediate this effect. We also show that mechanical stretch of cardiac myocytes downregulates Kv4.3 mRNA by the same pathway (Fig. 7). Finally, we established that this regulation is specific because Kv1.5 and Kv4.2 3’ UTRs are insensitive to Ang II. Thus, we have implicated physiological ROS signaling in long-term control of Kv4.3 expression and hence cardiac excitability.
Figure 7.
Induction of Kv4.3 mRNA destabilization in cardiac myocytes. The diagram on the left summarizes the conclusions from the signaling studies presented here, while chemical inhibitors and dominant negative subunits used are indicated on the right.
These findings have broad implications for function of the cardiovascular system. First, the AT1 receptor-stretch regulation of cardiac Kv4.3 expression could be activated in response to physiological and pathological changes in blood pressure. Second, since ASK1 is activated by a variety of reactive oxygen species (ROS)16,19, different ROS sources could trigger destabilization of Kv4.3 mRNA. For example, cardiac mitochondria generate ROS after reperfusion following ischemia. In addition, ROS generated by other cell types (i.e. endothelial and inflammatory cells) could diffuse into myocytes to affect the channel message. Therefore, the regulation described here could also be induced by ROS generated independently of AT1 receptors. Third, NADPH oxidase regulation of Kv4.3 may occur in other cell types. We did not detect this effect in neonatal cardiac fibroblasts, but these cells do not normally express Kv4.3. However, the rac-NADPH oxidase-p38 pathway has been found in Angiotensin-sensitive neurons in the brain and vascular smooth muscle20-23, and likewise, Kv4.3 channels are found in brain and smooth muscle15,24. Thus, NADPH oxidase could act in vascular smooth muscle cells and hypothalamic Angiotensin-sensitive neurons to suppress channel Kv4.3 expression. In each of these cases, this would be depolarizing and thus would promote hypertension. Finally, NADPH oxidase-induced mRNA destabilization may not be limited to the Kv4.3 transcript. We found that this regulatory mechanism does not affect the 3’ UTRs of Kv4.2 and Kv1.5. Since the former channel gene is regulated during cardiac hypertrophy in rodents (although not present in human ventricle), NADPH oxidase must not regulate stability of all transcripts associated with hypertrophy. Still, further study may reveal that Ang II-ROS-p38 signaling regulates 3’ UTR sequences of other mRNAs in the cardiovascular system.
Acknowledgments
We thank Dr. Elias Aizenman for his advice. This study was supported by NIH grants HL55312 and HL80632 to ESL and CIHR grant 3169 to AFRS.
References
- 1.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc. Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 2.Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res. 2005;96:1208–1216. doi: 10.1161/01.RES.0000170652.71414.16. [DOI] [PubMed] [Google Scholar]
- 3.Harris DM, Mills GD, Chen X, Kubo H, Berretta RM, Votaw VS, Santana LF, Houser SR. Alterations in early action potential repolarization causes localized failure of sarcoplasmic reticulum Ca2+ release. Circ Res. 2005;96:543–550. doi: 10.1161/01.RES.0000158966.58380.37. [DOI] [PubMed] [Google Scholar]
- 4.Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH. Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (I(to)) J Physiol. 2003;546:5–18. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. doi: 10.1161/01.cir.98.14.1383. [DOI] [PubMed] [Google Scholar]
- 6.Zicha S, Xiao L, Stafford S, Cha TJ, Han W, Varro A, Nattel S. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J Physiol. 2004;561:735–748. doi: 10.1113/jphysiol.2004.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takimoto K, Li D, Hershman KM, Li P, Jackson EK, Levitan ES. Decreased expression of Kv4.2 and novel Kv4.3 K+ channel subunit mRNAs in ventricles of renovascular hypertensive rats. Circ Res. 1997;81:533–539. doi: 10.1161/01.res.81.4.533. [DOI] [PubMed] [Google Scholar]
- 8.Zhang TT, Takimoto K, Stewart AF, Zhu C, Levitan ES. Independent regulation of cardiac Kv4.3 potassium channel expression by angiotensin II and phenylephrine. Circ Res. 2001;88:476–482. doi: 10.1161/01.res.88.5.476. [DOI] [PubMed] [Google Scholar]
- 9.Nakagami H, Takemoto M, Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2003;35:851–859. doi: 10.1016/s0022-2828(03)00145-7. [DOI] [PubMed] [Google Scholar]
- 10.Nishida M, Tanabe S, Maruyama Y, Mangmool S, Urayama K, Nagamatsu Y, Takagahara S, Turner JH, Kozasa T, Kobayashi H, Sato Y, Kawanishi T, Inoue R, Nagao T, Kurose H. G alpha 12/13- and reactive oxygen species-dependent activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase by angiotensin receptor stimulation in rat neonatal cardiomyocytes. J Biol Chem. 2005;280:18434–18441. doi: 10.1074/jbc.M409710200. [DOI] [PubMed] [Google Scholar]
- 11.Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, Baliga R, Wang J, Siwik DA, Singh K, Pagano P, Colucci WS, Sawyer DB. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res. 2001;89:453–460. doi: 10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- 12.Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem. 2003;278:31111–31117. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- 13.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 14.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serodio P, Vega-Saenz de Miera E, Rudy B. Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. J Neurophysiol. 1996;75:2174-2179. doi: 10.1152/jn.1996.75.5.2174. [DOI] [PubMed] [Google Scholar]
- 16.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig S, Hoffmeyer A, Goebeler M, Kilian K, Hafner H, Neufeld B, Han J, Rapp UR. The stress inducer arsenite activates mitogen-activated protein kinases extracellular signal-regulated kinases 1 and 2 via a MAPK kinase 6/p38-dependent pathway. J Biol Chem. 1998;273:1917–1922. doi: 10.1074/jbc.273.4.1917. [DOI] [PubMed] [Google Scholar]
- 19.Yasinska IM, Kozhukhar AV, Sumbayev VV. S-nitrosation of thioredoxin in the nitrogen monoxide/superoxide system activates apoptosis signal-regulating kinase 1. Arch Biochem Biophys. 2004;428:198–203. doi: 10.1016/j.abb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Bengtsson SH, Gulluyan LM, Dusting GJ, Drummond GR. Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. Clin Exp Pharmacol Physiol. 2003;30:849–854. doi: 10.1046/j.1440-1681.2003.03929.x. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 22.Sun C, Sellers KW, Sumners C, Raizada MK. NADPH oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res. 2005;96:659–666. doi: 10.1161/01.RES.0000161257.02571.4b. [DOI] [PubMed] [Google Scholar]
- 23.Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH Oxidase-Derived Superoxide Anion Mediates Angiotensin II-Induced Pressor Effect via Activation of p38 Mitogen-Activated Protein Kinase in the Rostral Ventrolateral Medulla. Circ Res. 2005 doi: 10.1161/01.RES.0000185804.79157.C0. in press. [DOI] [PubMed] [Google Scholar]
- 24.Ohya S, Tanaka M, Oku T, Asai Y, Watanabe M, Giles WR, Imaizumi Y. Molecular cloning and tissue distribution of an alternatively spliced variant of an A-type K+ channel alpha-subunit, Kv4.3 in the rat. FEBS Lett. 1997;420:47–53. doi: 10.1016/s0014-5793(97)01483-x. [DOI] [PubMed] [Google Scholar]