Abstract
The substance P (SP)-preferring receptor neurokinin-1 receptor (NK-1R) has two forms: a full-length receptor consisting of 407 aa and a truncated receptor consisting of 311 aa. These two receptors differ in the length of the C terminus of NK-1R. We studied the undifferentiated and phorbol myristate acetate (PMA)-differentiated human monocyte/macrophage cell line THP-1 to investigate the expression and function of NK-1R. The expression of full-length and truncated NK-1R in this cell line was determined by using real-time PCR and immunofluorescence staining. Undifferentiated THP-1 cells expressed only truncated NK-1R. The differentiation of THP-1 cells with PMA to a macrophage-like phenotype resulted in the expression of full-length NK-1R, which was functionally accompanied by an SP (10−6 M)-induced Ca2+ increase. In contrast, the addition of SP (10−6 M) did not trigger Ca2+ response in undifferentiated THP-1 cells; however, SP did enhance the CCR5-preferring ligand RANTES (CCL5)-mediated Ca2+ increase. When a plasmid containing the full-length NK-1R was introduced into undifferentiated THP-1 cells, exposure to SP triggered Ca2+ increase, demonstrating that the full-length NK-1R is required for SP-induced Ca2+ increase. The NK-1R antagonist aprepitant (Emend, Merck) inhibited both the SP-induced Ca2+ increase in PMA-differentiated THP-1 cells and the SP priming effect on the CCL5-mediated Ca2+ increase, indicating that these effects are mediated through the full-length and truncated NK-1R, respectively. Taken together, these observations demonstrate that there are unique characteristics of NK-1R expression and NK-1R-mediated signaling between undifferentiated THP-1 cells and THP-1 cells differentiated to the macrophage phenotype.
Keywords: CCL2/RANTES, substance P, calcium, aprepitant, RT-PCR
There is a functional interaction between the immune and nervous systems (1–3), and substance P (SP) is a major regulatory peptide in this interaction. SP elicits a wide variety of responses in human monocytes/macrophages. SP increases the functional phagocytic response in macrophages and other phagocytes (4). SP specifically activates NF-κB, a key transcriptional factor involved in the control of cytokine expression (5, 6). SP stimulates immune cells to produce inflammatory cytokines, including IL-1, IL-6, TNF-α (1), IFN-γ (7), and macrophage inflammatory protein 1β (8), which are important constituents of immune cell activation and act as physiological induction signals in the regulation of immune responses. SP also has a major role in inflammation, acting through the proinflammatory cytokine loop (9). SP concentrations are elevated at local sites of inflammation (10–12). For example, SP contributes to the inflammatory response mediated by respiratory syncytial virus infection, and this response is inhibited by treatment with anti-SP antibody (13). Thus, SP is a powerful immunomodulator and a critical link between the immune and nervous systems.
The biologic responses to SP are mediated by its preferring neurokinin-1 receptor (NK-1R). The human NK-1R gene (14–17), localized to chromosome 2, has been cloned. NK-1R has been identified on immune cells (1, 12, 18–22), including T and B lymphocytes (19, 23, 24), monocytes/macrophages (18, 25), neutrophils (26), and mast cells (27). In a mouse model, gene-targeted disruption of NK-1R protected the lung from immune complex injury, demonstrating that SP amplifies the inflammatory reaction through binding to NK-1R (28). Blocking NK-1R down-regulates CCR5 receptor expression in human monocyte-derived macrophages (29), suggesting that there is close interaction between NK-1R and β-chemokine receptors.
NK-1R is a member of the G protein-coupled receptor superfamily (Gq/11) with a typical structure of seven transmembrane domains, an extracellular N terminus, and an intracellular C terminus (14). A splice variant of the human NK-1R mRNA with a truncated C terminus (truncated NK-1R) has been cloned and identified (14). The full-length NK-1R is the predominant form expressed at sites in the human brain, whereas the truncated NK-1R is widespread throughout the central nervous system and in peripheral tissues (30). Both the long and short forms of NK-1R protein have been detected in rat submaxillary glands (31) and parotid (32). A distinct distribution of these two NK-1Rs has been observed in rat striatum, submaxillary glands, and patotid (32). The binding and signaling properties of the truncated receptor or mutants have been compared with those of the full-length receptor that is expressed in CHO cells, Xenopus oocytes, and KNRK cells (14, 33–35). Although the existence and expression of both the full-length and truncated NK-1R have been documented, the differences in their expression and physiological function, particularly in human monocytes/macrophages, have not been studied.
We examined the expression of full-length and truncated NK-1R in undifferentiated and phorbol myristate acetate (PMA)-differentiated THP-1 cells (a human monocyte/macrophage cell line) using real-time RT-PCR and immunofluorescence staining. We also examined functional activity of these two NK-1Rs by measuring the SP-induced Ca2+ increase, an important secondary messenger in G protein-coupled receptor signaling.
Results
Expression of Full-Length and Truncated NK-1R in Undifferentiated and PMA-Differentiated THP-1 Cells.
Using real-time RT-PCR, we examined the expression of full-length and truncated NK-1R mRNA in undifferentiated and PMA-differentiated THP-1 cells. The full-length NK-1R mRNA was not detected in undifferentiated THP-1; however, it was detected in PMA-differentiated THP-1 cells. On days 6, 9, and 12 after PMA treatment, the full-length NK-1R mRNA increased 4.01-, 4.81-, and 11.35-fold of the day-3 level in post-PMA-treated THP-1cells, respectively (Fig. 1A). The expression level of full-length NK-1R in day-12 post-PMA-treated THP-1 cells was significantly higher than that of undifferentiated THP-1 cells (P < 0.01) and that of day-3 post-PMA-treated THP-1 cells (P < 0.05). In contrast, both undifferentiated and PMA-differentiated THP-1 cells expressed truncated NK-1R mRNA, with no significant differences noted among the samples (Fig. 1B). The amplification of the full-length NK-1R plasmid served as a specificity control that was amplified only by the full-length NK-1R primer pair, but not by the truncated NK-1R primer pair (data not shown).
Fig. 1.
The effect of PMA differentiation of THP-1 cells on full-length and truncated NK-1R mRNA expression. (A) The effect of PMA differentiation of THP-1 on full-length NK-1R mRNA expression. ∗, The expression level of full-length NK-1R in day-12 post-PMA-treated THP-1 cells is significantly higher than that of undifferentiated (day 0; P < 0.01) and day-3 post-PMA differentiated cells (P < 0.05). (B) The effect of PMA differentiation of THP-1 cells on truncated NK-1R mRNA expression. The data represent the averages of three independent experiments.
We examined whether the differential expression of full-length NK-1R is detectable at the protein level by using immunofluorescence staining of PMA-differentiated THP-1 cells. Undifferentiated THP-1 cells stained positively only with an anti-N-terminal antibody (Fig. 2B), whereas PMA-differentiated THP-1 cells (day 12 post-PMA) were stained positively with both anti-N-terminal and anti-C-terminal antibodies (Fig. 2 C and D). Because the anti-C-terminal antibody detects the C-terminal tail that is missing from the truncated NK-1R, these results, in combination with the real-time RT-PCR results (Fig. 1), indicate that the undifferentiated THP-1 cells express only the truncated NK-1R, whereas the PMA-differentiated THP-1 cells express both the full-length and truncated NK-1R on the cell membrane.
Fig. 2.
Immunofluorescence staining of NK-1R in undifferentiated (A and B) and PMA-differentiated (C and D) THP-1 cells. Undifferentiated THP-1 cells stained positively only by anti-N-terminal antibody (B), but not by the anti-C-terminal antibody (A). PMA-differentiated THP-1 cells were stained positively by both anti-C-terminal (C) and anti-N-terminal (D) antibodies. (Magnification: ×400.) The data are representative of two independent experiments. Rabbit IgG was used as negative control (data not shown), which had the same background as the anti-C-terminal antibody-stained undifferentiated THP-1 cell.
Expression of Full-Length NK-1R Is Required for an SP-Induced Calcium Increase.
To determine whether the differential expression of these two forms of NK-1R has functional relevance, we tested the ability of SP to induce increases of cytosolic Ca2+ in undifferentiated and PMA-differentiated THP-1 cells. Treatment of the cells with SP (10−6 M) did not trigger calcium response in undifferentiated THP-1 cells (Fig. 3A). In contrast, the addition of SP to PMA-differentiated THP-1 cells led to increased cytosolic calcium concentration (from 50 nM to 160 nM) (Fig. 3B). This SP-induced increase in cytosolic Ca2+ was abrogated by preincubating the cells with the NK-1R antagonist aprepitant (2 × 10−5 M) for 30 min (Fig. 3B). To confirm that the expression of full-length NK-1R is required for an SP-induced calcium increase, we introduced a full-length NK-1R plasmid into undifferentiated THP-1 cells and examined whether introduction of the full-length NK-1R leads to responsiveness of the undifferentiated THP-1 cells to SP-induced Ca2+ response. The addition of SP (10−6 M) triggered the calcium response in the full-length NK-1R plasmid-transfected undifferentiated THP-1 cells, but not in control (nontransfected) THP-1 cells (Fig. 4). The results demonstrate that the expression of the full-length NK-1R is required for an SP-induced Ca2+ increase in undifferentiated THP-1 cells.
Fig. 3.
Effect of SP on the calcium increase in undifferentiated (A) and PMA-differentiated (B) THP-1 cells. (A) Undifferentiated THP-1 cells were treated with SP (10−6 M) as indicated by the arrow. (B) Twelve-day-differentiated THP-1 cells were preincubated in the presence or absence of aprepitant (2 × 10−5 M) for 30 min, and then SP (10−6 M) was added, as indicated by the arrow. The data shown are representative of three independent experiments.
Fig. 4.
Expression of full-length NK-1R is required for an SP-induced calcium increase in undifferentiated THP-1 cells. SP (10−6 M) induced calcium responses in undifferentiated THP-1 cells (A), and a full-length NK-1R gene-containing plasmid transfected undifferentiated THP-1 cells (B). The data shown are representative of three independent experiments.
Ca2+ Increase and Truncated NK-1R.
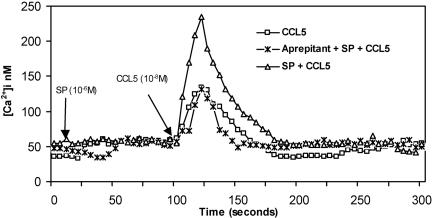
We determined whether the truncated NK-1R has a modulatory effect on β-chemokine Ca2+ signaling. The activation of truncated NK-1R modulated CCL5, RANTES (regulated upon activation, normal T cell-expressed and secreted)-induced calcium responses in fura-2 loaded undifferentiated THP-1 cells. Undifferentiated THP-1 cells were incubated in the presence or absence of aprepitant (2 × 10−5 M) for 30 min, pretreated with or without SP (10−6 M) for 90 s, and then stimulated with CCL5 (10−8 M) (Fig. 5). The resting calcium concentration was ≈50 nM. CCL5 triggered a rapid increase in cytosolic calcium to 140 nM, followed by a rapid decrease to basal levels. SP (10−6 M) by itself did not trigger a calcium response; however, the CCL5-induced cytosolic calcium concentration increased almost 2-fold in SP-pretreated THP-1 cells (240 nM). This effect of SP was completely abolished in aprepitant (Emend, 2 × 10−5 M)-pretreated undifferentiated THP-1 cells. The CCL5-induced calcium increase, however, was not affected by aprepitant pretreatment. Thus, the ability of SP to enhance a CCL5-triggered response was mediated through the activation of the truncated NK-1R, because the undifferentiated THP-1 cells express only the truncated NK-1R (Figs. 1 and 2). SP enhancement was completely abrogated by aprepitant pretreatment of these cells. Therefore, the truncated NK-1R is functional in THP-1 cells and has a positive modulatory effect on the CCL5-induced calcium increase.
Fig. 5.
SP enhanced the CCL5-mediated calcium response via truncated NK-1R. Undifferentiated THP-1 cells were incubated in the presence or absence of aprepitant (2 × 10−5 M) for 30 min before the addition of buffer or SP (10 s), followed by CCL5 at 90 s. Addition of SP (10−6 M) at 10 s and CCL5 (10−8 M) at 100 s is indicated by arrows. The data shown are representative of three independent experiments.
Discussion
NK-1R mRNA is expressed at a very low level in primary human peripheral monocytes and monocyte-derived macrophages, (18, 36, 37). SP-induced calcium mobilization has not been previously reported in primary human peripheral blood monocytes or monocyte-derived macrophages to our knowledge. However, there are many important observations related to the expression and function of full-length and truncated NK-1R in cell lines, including Xenopus oocytes and COS cells (14, 35), human IM-9 lymphoblasts (38, 39), the T lymphocyte cell line Jurkat (40), THP-1 cells (41), CHO cells (34, 42), HEK293 cells (43), and KNRK cells (33, 44–47). We used the THP-1 human monocyte/macrophage cell line initially derived from the peripheral blood of a patient with monocytic leukemia (48) to study the differential expression and functional difference between the full-length and truncated NK-1R in undifferentiated and PMA-differentiated THP-1 cells. THP-1 expresses NK-1R (41) and differentiates into macrophages after PMA treatment (49). Undifferentiated THP-1 cells expressed only the truncated NK-1R, whereas the differentiated THP-1 cells, induced by PMA to a macrophage-like lineage, expressed the full-length NK-1R in addition to the truncated form. Our investigation demonstrates that there is a differential expression of full-length and truncated NK-1R associated with cell differentiation and observes different functional consequences with the differential expression of these two forms of NK-1R. The expression of the full-length NK-1R is required for an SP-induced Ca2+ increase (Figs. 1–4). Treatment with SP did not trigger calcium response in undifferentiated THP-1 cells, which express only the truncated NK-1R. Treatment with SP, however, primes the CCL5-mediated calcium increase triggered through the truncated NK-1R. The SP effects are both NK-1R-specific because they are inhibited by an NK-1R antagonist (aprepitant, Emend). Our data provide evidence demonstrating that the full-length NK-1R has a complete signal pathway that includes the increase of calcium, whereas the truncated NK-1R has only an incomplete signal pathway, attributable to its lack of the C-terminal tail of NK-1R. Although the truncated NK-1R is sufficient to prime the β-chemokine receptors, it lacks the capacity to mobilize calcium in response to SP activation. The observation that the activation of truncated NK-1R primes CCL5-mediated calcium response is evidence for a new endogenous linkage between the neural and immune systems and suggests a possible role of SP in regulation of β-chemokine receptor function in monocytes/macrophages. THP-1 cells are an excellent cell model to investigate those factors that participate in the regulation of the differential expression of these two forms of NK-1R.
The full-length NK-1R expression was significantly enhanced by PMA treatment, but the truncated NK-1R expression was not altered by PMA (Fig. 1). PMA, a PKC-activating phorbol ester, is an effective inducer of monocyte/macrophage differentiation in myeloid cell lines in vitro (50). PMA-induced differentiation is accompanied by morphological and functional changes including increased adherence, cessation of cell proliferation, and increased phagocytic capability, as well as increased expression of CD11a and CD11b (restricted to mature myeloid cells) and decreased expression of c-myc and c-myb oncogenes. In addition, other gene expression is altered after PMA treatment. For example, two K+ channels were observed in THP-1 cells only after differentiation into macrophages (51). Truncated NK-1R has been observed (14, 30, 32, 52); however, its regulation in human immune cells has not previously been investigated. Our observed selective effect of PMA on the full-length NK-1R demonstrates a role for NK-1R in macrophage differentiation, yet the mechanism is unknown.
The full-length NK-1R, but not the truncated form, triggers an increase of calcium in THP-1 cells (Figs. 1, 3, and 4), indicating that the C-terminal sequence of NK-1R is required for SP-induced calcium response. NK-1R mRNA is expressed at a very low level in human monocytes/macrophages (18, 36, 37), and the truncated NK-1R mRNA was the only form detected in human macrophages (30). NK-1R-mediated calcium increases have not been demonstrated in human primary monocytes/macrophages. Elevations in cytosolic free Ca2+ occur in cell lines transfected with full-length NK-1R plasmid after exogenous treatment with SP (40, 45, 53). Using a rat full-length NK-1R stably transfected human lymphocyte cell line (Jurkat/SPR), Sudduth-Klinger et al. (40) showed that SP induced a calcium increase in a dose-dependent fashion ranging from 10−11 to 10−6 M. The response was maximal at 10−7 M, with an EC50 of 0.3–0.5 × 10−9 M (40). A higher concentration of SP, however, is required to induce calcium mobilization in primary cell cultures. For example, in a study of functional NK-1R activity in bovine ovarian macrophages, Brylla et al. (54) observed that SP induced a calcium increase at 10−6 M (in 75% of cells) and 10−7 M (in 18.5% of cells) but did not induce a calcium increase at 10−8 M. In cultured rabbit osteoclasts, the concentration range of the SP-induced calcium increase was 3 × 10−7 M to 5 × 10−6 M, with an EC50 of 2 × 10−7 M (55). In our study of PMA-differentiated THP-1, we observed a calcium increase induced by SP (10−6 M), yet there were no obvious increases of intracellular calcium when 10−7 M SP was used (data not shown). Because the full-length NK-1R expression is required for an SP-induced calcium increase in THP-1 cells (Figs. 3 and 4), we postulate that, if an SP-induced calcium increase is not detectable in certain cell systems, this is most likely an indication of the absence of full-length NK-1R expression in those systems (such as undifferentiated THP-1 cells); if higher SP concentrations are needed to induce a calcium increase in a given cell system (such as some primary cells and cell lines, including PMA-differentiated THP-1 cells) than that of NK-1R plasmid transfected cell lines, then that is a reflection of much lower levels of full-length NK-1R expression in that particular cell system in comparison to NK-1R plasmid-transfected cell lines. Furthermore, Marriott et al. (56) have shown that SP did not increase intracellular calcium even at 10−6 M concentration in murine macrophages and dendritic cells, although they demonstrated that these cells express NK-1R (57). When a full-length NK-1R gene-containing plasmid was introduced into freshly isolated human monocytes, however, SP did stimulate a calcium increase (data not shown). In addition, Nowak et al. (58) demonstrated an SP-induced increase in intracellular free calcium concentration in human polymorphonuclear neutrophils in 40% (4 of 10) of donors studied. The finding indicates donor variability in response to SP-induced calcium responses. The observed lack of SP-induced calcium response in mouse macrophages (56) and the response variations among donors observed in neutrophils (58) may be caused by the absence of the full-length NK-1R expression and donor variability in the expression of full-length NK-1R, respectively.
The only sequence difference between the two forms of NK-1R is in the length of the C-terminal tail. NK-1R is coupled to the pertussis toxin-insensitive Gq/11 family, which is linked to phosphoinositide metabolism (59). In general, in the Gq/11 family, the G protein in its “resting state” is linked to one of the intracellular loops of the receptor. After ligand–receptor interaction, conformation changes within the receptor cause the G protein to exchange GDP for GTP. This exchange activates the G protein, causing dissociation of the α-subunit, which binds to and activates the intracellular effector. The intracellular effector is phospholipase C. Activation of NK-1R initiates phosphoinositol breakdown, leading to the production of inositol 1,4,5-trisphosphate and diacylglycerol. Inositol 1,4,5-trisphosphate releases calcium from intracellular stores, and diacylglycerol increases intracellular calcium by opening voltage-gated Ca2+ channels via PKC (60). The truncated receptor couples to calcium mobilization in transfected KNRK and CHO cells (33, 34). These studies, however, are not inconsistent with our finding that the truncated NK-1R does not couple to calcium mobilization in monocytes/macrophages (THP-1 cells). In the studies of KNRK cells, the C-terminal truncated NK-1R plasmids reported to couple to calcium mobilization were 324, 342, and 354 aa in length. The NK-1R of the 311-aa form was not reported (33). The truncated NK-1R of the 311-aa form corresponds to the naturally truncated, potentially alternatively spliced NK-1R short form (14). In studies examining the role of the C-terminal tail of rat NK-1R with progressively shorter mutants with 16, 47, 70, and 96 amino acid residues deleted from the tail, respectively, Sasakawa et al. (35) observed that in Xenopus oocytes the mutants D96 (which corresponded to the natural truncated, alternatively spliced variant, 311 aa) and D70 (337 aa) had a reduced maximum current response (SP-induced Ca2+-dependent Cl− current response) (only 5% and 70% of the full-length NK-1R, respectively) in response to SP in comparison to the full-length NK-1R (35). Other mutants, D16 and D47, however, exhibited a normal current response consistent with findings for truncated NK-1R expressed in KNRK cells reported by Bohm et al. (33). Furthermore, weak or no responsiveness of the naturally truncated NK-1R to SP was also observed in another study examining the function of human full-length and truncated NK-1R (14). Based on these studies (14, 33–35) and our observation, we conclude that the domain between 311 and 324/337 amino acid residues has a crucial role for NK-1R function(s) such as calcium mobilization. In the absence of this domain, SP will not induce calcium mobilization. This conclusion has also been confirmed in a comparison of the differences in SP-induced calcium mobilization between the full-length and the truncated NK-1R (311 aa) plasmid-transfected HEK293 cells (unpublished observation). These studies support the important role of the C-terminal tail in NK-1R function and suggest that the presence of the C-terminal tail is the structural basis for an SP-induced calcium increase.
Although SP did not induce a calcium increase via the truncated NK-1R (Fig. 3), it primes a CCL5-mediated calcium increase (Fig. 5), indicating that the truncated NK-1R also has functional activity. This priming effect was NK-1R-dependent, because treatment of the cells with the NK-1R antagonist aprepitant abrogated the priming effect. Thus, there is interaction between truncated NK-1R and β-chemokine receptors, and that activation of the truncated NK-1R modulates β-chemokine receptor responses. Enhanced intracellular Ca2+ response resulting from the interaction between different G protein-coupled receptors (priming effect or sensitization) is a common phenomenon [for review, see Werry et al. (61)]. These interactions have important implications for the control of cell function. Although the specific mechanisms of SP priming of the β-chemokine receptor remains unknown, the finding that truncated NK-1R interacts with β-chemokine receptors affords an approach to investigation of the role of SP in the regulation of β-chemokine receptor function in monocytes/macrophages and regulation of immune function in physiological and pathological situations.
In summary, NK-1R expression and function are different in undifferentiated THP-1 cells in comparison to THP-1 cells differentiated to a macrophage phenotype. The observed functional differences between full-length and truncated NK-1R offer insights into the differences in signaling pathways (Ca2+-dependent) and the mechanisms by which the truncated NK-1R interacts with β-chemokine receptors in mononuclear phagocytes.
Materials and Methods
Cells and PMA-Induced Differentiation.
The human monocytic cell line THP-1 (48) was obtained from the American Type Culture Collection. THP-1 cells, in suspension, were maintained at 37°C under 5% CO2 in RPMI medium 1640 supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. THP-1 cells were induced to differentiate into adherent macrophage-like cells by treatment with PMA (Sigma–Aldrich). Cells were plated at 3 × 105 cells per well in 24-well plates or at 5 × 105 cells per well in 6-well plates and treated with 16 nM PMA for up to 12 days at 37°C. After treatment with PMA, differentiation occurs, and the cells adhere to the plates and become flat and amoeboid in shape.
RNA Extraction.
Total RNA was extracted from undifferentiated and PMA-differentiated THP-1 cells by using Tri-Reagent (Molecular Research Center, Cincinnati), as instructed by the manufacturer. After centrifugation at 13,000 × g for 15 min, RNA-containing aqueous phase was precipitated in isopropanol. RNA precipitates then were washed once in 75% ethanol and solubilized in 30 μl of RNase-free water.
Real-Time PCR Primers.
The PCR primers used for quantitative measurement of the long and short isoforms of NK-1R mRNA were modified from Caberlotto et al. (30). The sequences of the primer pair used to amplify the long isoform of NK-1R were 5′-TCTTCTTCCTCCTGCCCTACATC-3′ (sense) and 5′-AGCACCGGAAGGCATGCTTGAAGCCCA-3′ (antisense), which is specific for the full-length NK-1R sequence. The sequences of the primer pair for the truncated NK-1R were 5′-TCTTCTTCCTCCTGCCCTACATC-3′ (sense) and 5′-TGGAGAGCTCATGGGGTTGGGATCCT-3′ (antisense). The sequences of the primer pair for GAPDH were 5′-GGTGGTCTCCTCTGACTTCAACA-3′ (sense) and 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ (antisense) (62). The primers were resuspended in Tris-EDTA buffer and stored at −30°C.
Reverse Transcription.
Total RNA (1 μg) was subjected to reverse transcription. The final reaction mixture (20 μl) contained the following elements: 5 mM MgCl2, 1× reverse transcription buffer, 500 μM each dNTP, 1 unit/μl recombinant RNasin, 10–15 units of avian myeloblastosis virus reverse transcriptase (Promega), and 50 ng of random primers. Reverse transcription was performed at 42°C for 1 h. The reaction was terminated by holding the reaction mixture at 99°C for 5 min. One-tenth (2 μl) of the resulting cDNA was used as a template for real-time PCR amplification.
Real-Time PCR Assay.
The iQ iCycler system (Bio-Rad) was used for real-time PCR analysis. Thermal cycling conditions were designed as follows: initial denaturation at 95°C for 3 min followed by 40–45 cycles of 95°C for 15 s and 60°C for 1 min. Fluorescent measurements were recorded during each annealing step. At the end of each PCR run, data were automatically analyzed by the system, and amplification plots were obtained. For each PCR, 2 μl of cDNA template was added to 23 μl of iQ SYBR Supermix (Bio-Rad) containing the primer pair for either the full-length or the truncated NK-1R, or for GAPDH. An NK-1R full-length plasmid (a gift from Norma Gerard, Harvard University, Boston) was used as a specificity control for real-time PCR. This plasmid was amplified by the primer pair for the full-length NK-1R but not by the primer pair for the truncated NK-1R. All amplification reactions were performed in duplicate, and average threshold cycle (Ct) numbers of the duplicates were used to calculate the relative mRNA expression of full-length and truncated NK-1R. To control the integrity of the RNA and normalize NK-1R mRNA levels in these samples, a GAPDH mRNA fragment in the cells also was amplified by using our established real-time RT-PCR with iQ SYBR green Supermix (Bio-Rad), as reported (62). The standard comparative Ct method was used to determine the relative NK-1R gene expression levels (63). Ct values of the full-length and truncated NK-1R cDNA were normalized to an endogenous cDNA, GAPDH. The magnitude of change of the full-length and truncated NK-1R mRNA expression in PMA-differentiated THP-1 cells in comparison to undifferentiated THP-1 cells was calculated by using the standard 2−(ΔΔCt) method (63). Each Ct represents the mean Ct value for duplicate samples.
Immunofluorescence Staining of NK-1R in THP-1 and PMA-Differentiated THP-1.
Differential expression of human full-length and truncated NK-1R was evaluated by using an anti-N-terminal of NK-1R antibody (Novus Biologicals, Littleton, CO) and an anti-C-terminal of NK-1R antibody (Santa Cruz Biotechnology). The anti-N-terminal antibody detects both full-length and truncated NK-1R because it was produced against a synthetic peptide corresponding to the extracellular N-terminal amino acids 1–17 of human NK-1R. The anti-C-terminal antibody (Santa Cruz Biotechnology) detects only the full-length NK-1R because it was raised against a recombinant protein that corresponds to amino acid residues 325–407, which are absent in the truncated NK-1R (14).
PMA-differentiated THP-1 cells were maintained on 12-mm glass coverslips in a 24-well plate, and undifferentiated THP-1 cells were maintained in suspension. PMA-differentiated THP-1 cells (day-12 post-PMA treatment) and undifferentiated THP-1 cells were washed three times in 0.1 M PBS and fixed in 2% paraformaldehyde in PBS for 10 min. After additional washes, the cells were pretreated with a blocking solution (minimal essential medium) containing 15 mM Hepes buffer, 10% FBS, 10% normal goat serum, and 0.05% sodium azide for 10 min. These cells then were incubated with either normal rabbit IgG (1:1,000, 1 μg/ml) or primary antibodies (anti-N-terminal or anti-C-terminal of NK-1R) diluted in the blocking solution at room temperature for 1 h. The secondary antibody, goat anti-rabbit IgG, was conjugated to FITC (1:100; Southern Biotechnology Associates). The cells were washed in PBS and mounted in Vectashield (Vector Laboratories). The samples were examined and imaged by using a conventional fluorescence microscope. Digital images were captured in black and white, then pseudocolored by using spot v.4.0.1 software (Diagnostic Instruments, Sterling Heights, MI). Figures were assembled by using photoshop 7.0 (Adobe Systems, San Jose, CA).
Calcium Increase.
Undifferentiated THP-1 cells (2 × 106 cells) were plated on poly-d-lysine-coated glass coverslips (Sigma) in six-well plates. PMA-differentiated THP-1 cells were cultured on coverslips (Fisher Scientific) in six-well plates. The coverslip was sized to fit a homeothermic perfusion chamber platform of an inverted Zeiss microscope. The cells (undifferentiated and day 12 after PMA treatment) were loaded with 2 μM fura-2 acetoxymethylester (Molecular Probes) in the presence of 0.2 mg/ml pluronic F-127 (Molecular Probes) in 3 ml of Hanks’ buffered salt solution supplemented with 1% FBS and 1.25 mM CaCl2 for 30 min at 37°C. Pretreatment of the cells for 30 min with buffer or 2 × 10−5 M aprepitant (Emend), an NK-1R antagonist that blocks SP binding to NK-1R, was performed at the same time as the loading process. Aprepitant (Emend) was purchased from Merck through our hospital pharmacy. The cells then were superfused with Hanks’ buffered salt solution supplemented with 1% FBS and 1.25 mM CaCl2 at 37°C in the presence or absence of SP (10−6 M) and/or addition of CCL5 (10−8 M, added at 100 s and recorded up to 300 s). The flow rate was 1.5 ml/min, and excitation was performed at 334 and 380 nm with two narrow band-pass filters. The emitted fluorescence was filtered (520 nm), captured with an Attofluor charge-coupled device video camera (512 × 480 pixel resolution), digitized (256 gray levels), and analyzed with attofluor ratio vision 6.00 software (Atto Instruments, Rockville, MD). acellular Ca2+ concentration was calculated by comparing the ratio of fluorescence at each pixel to an in vitro two-point caliburve. The Ca2+ concentration presented is that obtained by averaging the values of all pixels of a cell body. The data points were collected at 5-s intervals.
Transfection of NK-1R by Nucleofection.
Introduction of the full-length NK-1R plasmid into THP-1 cells was performed by using Nucleofector according to the manufacturer’s instructions (Amaxa, Gaithersburg, MD). In brief, THP-1 cells were split 1 day before nucleofection and grown at a density of 5 × 105 cells per ml. For each transfe × 106 cells were suspended in 100 μl of Nucleofector Solution. NK-1R full-length gene-containing plasmid (0.5 μg) was used for each nucleofection. The Cell Line Nucleofection Kit V was used following the manufacturer’s protocol. Transfected cells then were transferred to six-well plates and incubated for 48 h befuring the Ca2+ increase.
Statistical Analysis.
instat 3.0 software (GraphPad) was used for statistical analysis. One-way ANOVA was used to analyze the expression of full-length and truncated NK-1R mRNA in undifferentiated and PMA-differentiated THP-1 cells.
Acknowledgments
We thank Yan-Jian Wang, Chang-Jiang Guo, Chun Ho, and Stephen Jasionowski for their work on the manuscript. This investigation was supported by National Institutes of Health Grants MH49981 and P01-MH76388 (to S.D.D.), DA12815 (to W.Z.H.), GM64552 (to L.E.K.), and AI24840 (to H.M.K.).
Abbreviations
- SP
substance P
- NK-1R
neurokinin-1 receptor
- PMA
phorbol myristate acetate.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Ho W. Z., Douglas S. D. J. Neuroimmunol. 2004;157:48–55. doi: 10.1016/j.jneuroim.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Black I. B., Adler J. E., Dreyfus C. F., Jonakait G. M., Katz D. M., LaGamma E. F., Markey K. M. Science. 1984;225:1266–1270. doi: 10.1126/science.6147894. [DOI] [PubMed] [Google Scholar]
- 3.McGillis J. P., Mitsuhashi M., Payan D. G. Ann. N.Y. Acad. Sci. 1990;594:85–94. doi: 10.1111/j.1749-6632.1990.tb40470.x. [DOI] [PubMed] [Google Scholar]
- 4.Hartung H. P. Brain Behav. Immun. 1988;2:275–281. doi: 10.1016/0889-1591(88)90029-3. [DOI] [PubMed] [Google Scholar]
- 5.Lieb K., Fiebich B. L., Berger M., Bauer J., Schulze-Osthoff K. J. Immunol. 1997;159:4952–4958. [PubMed] [Google Scholar]
- 6.Marriott I., Bost K. L. J. Immunol. 2000;165:182–191. doi: 10.4049/jimmunol.165.1.182. [DOI] [PubMed] [Google Scholar]
- 7.Blum A. M., Metwali A., Elliott D. E., Weinstock J. V. Am. J. Physiol. 2003;284:G197–G204. doi: 10.1152/ajpgi.00271.2002. [DOI] [PubMed] [Google Scholar]
- 8.Guo C. J., Lai J. P., Luo H. M., Douglas S. D., Ho W. Z. J. Neuroimmunol. 2002;131:160–167. doi: 10.1016/s0165-5728(02)00277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Payan D. G. Annu. Rev. Med. 1989;40:341–352. doi: 10.1146/annurev.me.40.020189.002013. [DOI] [PubMed] [Google Scholar]
- 10.Payan D. G., McGillis J. P., Goetzl E. J. Adv. Immunol. 1986;39:299–323. doi: 10.1016/s0065-2776(08)60353-3. [DOI] [PubMed] [Google Scholar]
- 11.Bost K. L. Front. Biosci. 2004;9:1994–1998. doi: 10.2741/1376. [DOI] [PubMed] [Google Scholar]
- 12.Bost K. L. Front. Biosci. 2004;9:3331–3332. doi: 10.2741/1484. [DOI] [PubMed] [Google Scholar]
- 13.Tripp R. A., Dakhama A., Jones L. P., Barskey A., Gelfand E. W., Anderson L. J. J. Virol. 2003;77:6580–6584. doi: 10.1128/JVI.77.11.6580-6584.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong T. M., Anderson S. A., Yu H., Huang R. R., Strader C. D. Mol. Pharmacol. 1992;41:24–30. [PubMed] [Google Scholar]
- 15.Gerard N. P., Garraway L. A., Eddy R. L., Jr., Shows T. B., Iijima H., Paquet J. L., Gerard C. Biochemistry. 1991;30:10640–10646. doi: 10.1021/bi00108a006. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins B., Powell S. J., Danks P., Briggs I., Graham A. Biochem. Biophys. Res. Commun. 1991;180:1110–1117. doi: 10.1016/s0006-291x(05)81181-7. [DOI] [PubMed] [Google Scholar]
- 17.Takeda Y., Chou K. B., Takeda J., Sachais B. S., Krause J. E. Biochem. Biophys. Res. Commun. 1991;179:1232–1240. doi: 10.1016/0006-291x(91)91704-g. [DOI] [PubMed] [Google Scholar]
- 18.Ho W. Z., Lai J. P., Zhu X. H., Uvaydova M., Douglas S. D. J. Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- 19.Lai J. P., Douglas S. D., Ho W. Z. J. Neuroimmunol. 1998;86:80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 20.Lai J. P., Douglas S. D., Rappaport E., Wu J. M., Ho W. Z. J. Neuroimmunol. 1998;91:121–128. doi: 10.1016/s0165-5728(98)00170-2. [DOI] [PubMed] [Google Scholar]
- 21.Lai J. P., Zhan G. X., Campbell D. E., Douglas S. D., Ho W. Z. Neuroscience. 2000;101:1137–1144. doi: 10.1016/s0306-4522(00)00398-5. [DOI] [PubMed] [Google Scholar]
- 22.Lai J. P., Douglas S. D., Zhao M., Ho W. Z. J. Immunol. Methods. 1999;230:149–157. doi: 10.1016/s0022-1759(99)00120-9. [DOI] [PubMed] [Google Scholar]
- 23.Payan D. G., Brewster D. R., Missirian-Bastian A., Goetzl E. J. J. Clin. Invest. 1984;74:1532–1539. doi: 10.1172/JCI111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanisz A. M., Scicchitano R., Dazin P., Bienenstock J., Payan D. G. J. Immunol. 1987;139:749–754. [PubMed] [Google Scholar]
- 25.Lucey D. R., Novak J. M., Polonis V. R., Liu Y., Gartner S. Clin. Diagn. Lab. Immunol. 1994;1:330–335. doi: 10.1128/cdli.1.3.330-335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wozniak A., McLennan G., Betts W. H., Murphy G. A., Scicchitano R. Immunology. 1989;68:359–364. [PMC free article] [PubMed] [Google Scholar]
- 27.Shanahan F., Denburg J. A., Fox J., Bienenstock J., Befus D. J. Immunol. 1985;135:1331–1337. [PubMed] [Google Scholar]
- 28.Bozic C. R., Lu B., Hopken U. E., Gerard C., Gerard N. P. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- 29.Lai J. P., Ho W. Z., Zhan G. X., Yi Y., Collman R. G., Douglas S. D. Proc. Natl. Acad. Sci. USA. 2001;98:3970–3975. doi: 10.1073/pnas.071052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caberlotto L., Hurd Y. L., Murdock P., Wahlin J. P., Melotto S., Corsi M., Carletti R. Eur. J. Neurosci. 2003;17:1736–1746. doi: 10.1046/j.1460-9568.2003.02600.x. [DOI] [PubMed] [Google Scholar]
- 31.Kage R., Leeman S. E., Boyd N. D. J. Neurochem. 1993;60:347–351. doi: 10.1111/j.1471-4159.1993.tb05857.x. [DOI] [PubMed] [Google Scholar]
- 32.Mantyh P. W., Rogers S. D., Ghilardi J. R., Maggio J. E., Mantyh C. R., Vigna S. R. Brain Res. 1996;719:8–13. doi: 10.1016/0006-8993(96)00050-9. [DOI] [PubMed] [Google Scholar]
- 33.Bohm S. K., Khitin L. M., Smeekens S. P., Grady E. F., Payan D. G., Bunnett N. W. J. Biol. Chem. 1997;272:2363–2372. doi: 10.1074/jbc.272.4.2363. [DOI] [PubMed] [Google Scholar]
- 34.Li H., Leeman S. E., Slack B. E., Hauser G., Saltsman W. S., Krause J. E., Blusztajn J. K., Boyd N. D. Proc. Natl. Acad. Sci. USA. 1997;94:9475–9480. doi: 10.1073/pnas.94.17.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasakawa N., Sharif M., Hanley M. R. FEBS Lett. 1994;347:181–184. doi: 10.1016/0014-5793(94)00532-x. [DOI] [PubMed] [Google Scholar]
- 36.Goode T., O’Connell J., Ho W. Z., O’Sullivan G. C., Collins J. K., Douglas S. D., Shanahan F. Clin. Diagn. Lab. Immunol. 2000;7:371–376. doi: 10.1128/cdli.7.3.371-376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai J. P., Douglas S. D., Wang Y. J., Ho W. Z. Clin. Diagn. Lab. Immunol. 2005;12:537–541. doi: 10.1128/CDLI.12.4.537-541.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Payan D. G., McGillis J. P., Organist M. L. J. Biol. Chem. 1986;261:14321–14329. [PubMed] [Google Scholar]
- 39.McGillis J. P., Organist M. L., Scriven K. H., Payan D. G. J. Neurosci. Res. 1987;18:190–194. doi: 10.1002/jnr.490180127. [DOI] [PubMed] [Google Scholar]
- 40.Sudduth-Klinger J., Schumann M., Gardner P., Payan D. G. Cell. Mol. Neurobiol. 1992;12:379–395. doi: 10.1007/BF00711540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simeonidis S., Castagliuolo I., Pan A., Liu J., Wang C. C., Mykoniatis A., Pasha A., Valenick L., Sougioultzis S., Zhao D., Pothoulakis C. Proc. Natl. Acad. Sci. USA. 2003;100:2957–2962. doi: 10.1073/pnas.0530112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacDonald D., Silberman S. C., Lowe J. A., III, Drozda S. E., Leeman S. E., Boyd N. D. Mol. Pharmacol. 1996;49:808–813. [PubMed] [Google Scholar]
- 43.Richardson M. D., Balius A. M., Yamaguchi K., Freilich E. R., Barak L. S., Kwatra M. M. J. Neurochem. 2003;84:854–863. doi: 10.1046/j.1471-4159.2003.01577.x. [DOI] [PubMed] [Google Scholar]
- 44.Mitsuhashi M., Ohashi Y., Shichijo S., Christian C., Sudduth-Klinger J., Harrowe G., Payan D. G. J. Neurosci. Res. 1992;32:437–443. doi: 10.1002/jnr.490320315. [DOI] [PubMed] [Google Scholar]
- 45.Christian C., Gilbert M., Payan D. G. Neuroimmunomodulation. 1994;1:159–164. doi: 10.1159/000097156. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert M. S., Bunnett N. W., Payan D. G. Cell. Mol. Neurobiol. 1992;12:529–545. doi: 10.1007/BF00711233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vigna S. R., Bowden J. J., McDonald D. M., Fisher J., Okamoto A., McVey D. C., Payan D. G., Bunnett N. W. J. Neurosci. 1994;14:834–845. doi: 10.1523/JNEUROSCI.14-02-00834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Int. J. Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 49.Asseffa A., Dickson L. A., Mohla S., Bremner T. A. Oncol. Res. 1993;5:11–18. [PubMed] [Google Scholar]
- 50.Matikainen S., Hurme M. Int. J. Cancer. 1994;57:98–103. doi: 10.1002/ijc.2910570118. [DOI] [PubMed] [Google Scholar]
- 51.DeCoursey T. E., Kim S. Y., Silver M. R., Quandt F. N. J. Membr. Biol. 1996;152:141–157. doi: 10.1007/s002329900093. [DOI] [PubMed] [Google Scholar]
- 52.Baker S. J., Morris J. L., Gibbins I. L. Brain Res. Mol. Brain Res. 2003;111:136–147. doi: 10.1016/s0169-328x(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 53.Seabrook G. R., Fong T. M. Neurosci. Lett. 1993;152:9–12. doi: 10.1016/0304-3940(93)90470-6. [DOI] [PubMed] [Google Scholar]
- 54.Brylla E., Aust G., Geyer M., Uckermann O., Loffler S., Spanel-Borowski K. Regul. Pept. 2005;125:125–133. doi: 10.1016/j.regpep.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Mori T., Ogata T., Okumura H., Shibata T., Nakamura Y., Kataoka K. Biochem. Biophys. Res. Commun. 1999;262:418–422. doi: 10.1006/bbrc.1999.1220. [DOI] [PubMed] [Google Scholar]
- 56.Marriott I., Mason M. J., Elhofy A., Bost K. L. J. Neuroimmunol. 2000;102:163–171. doi: 10.1016/s0165-5728(99)00182-4. [DOI] [PubMed] [Google Scholar]
- 57.Marriott I., Bost K. L. J. Neuroimmunol. 2001;114:131–141. doi: 10.1016/s0165-5728(00)00466-5. [DOI] [PubMed] [Google Scholar]
- 58.Nowak D., Hrabec E., Greger J., Piasecka G., Krol M., Bialasiewicz P., Antczak A., Plucienniczak G., Plucienniczak A. Int. J. Clin. Lab. Res. 1996;26:106–111. doi: 10.1007/BF02592352. [DOI] [PubMed] [Google Scholar]
- 59.Khawaja A. M., Rogers D. F. Int. J. Biochem. Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- 60.Gallacher D. V., Hanley M. R., Petersen O. H., Roberts M. L., Squire-Pollard L. G., Yule D. I. J. Physiol. 1990;426:193–207. doi: 10.1113/jphysiol.1990.sp018133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Werry T. D., Wilkinson G. F., Willars G. B. Biochem. J. 2003;374:281–296. doi: 10.1042/BJ20030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lai J. P., Yang J. H., Douglas S. D., Wang X., Riedel E., Ho W. Z. Clin. Diagn. Lab. Immunol. 2003;10:1123–1128. doi: 10.1128/CDLI.10.6.1123-1128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Livak K. J., Schmittgen T. D. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]