Abstract
Higher yields and reduced pesticide impacts are needed to mitigate the effects of agricultural intensification. A 2-year farm-scale evaluation of 81 commercial fields in Arizona show that use of transgenic Bacillus thuringiensis (Bt) cotton reduced insecticide use, whereas transgenic cotton with Bt protein and herbicide resistance (BtHr) did not affect herbicide use. Transgenic cotton had higher yield than nontransgenic cotton for any given number of insecticide applications. However, nontransgenic, Bt and BtHr cotton had similar yields overall, largely because higher insecticide use with nontransgenic cotton improved control of key pests. Unlike Bt and BtHr cotton, insecticides reduced the diversity of nontarget insects. Several other agronomic and ecological factors also affected biodiversity. Nevertheless, pairwise comparisons of diversity of nontarget insects in cotton fields with diversity in adjacent noncultivated sites revealed similar effects of cultivation of transgenic and nontransgenic cotton on biodiversity. The results indicate that impacts of agricultural intensification can be reduced when replacement of broad-spectrum insecticides by narrow-spectrum Bt crops does not reduce control of pests not affected by Bt crops.
Keywords: agricultural sustainability, environmental impact, transgenic crops
The increasing world population and changes in consumption patterns may necessitate significant agricultural intensification in the next 50 years (1, 2). Unless crop yield is improved and release of fertilizers and pesticides from croplands is reduced, such intensification could augment contamination and perturbation of managed and natural ecosystems, ultimately harming biodiversity and public health (1–4). It was proposed that transgenic Bacillus thuringiensis (Bt) crops could be valuable tools for increasing agricultural productivity while minimizing the environmental impacts of agriculture (1, 2). However, the potential effects of transgenic crops on nontarget arthropods have caused concern, especially in regions where agricultural land is important to sustain biodiversity (5–7).
Although Bt crops are grown extensively worldwide (8), no large-scale studies had been performed to simultaneously test whether they have favorable agricultural effects and minimal impacts on nontarget arthropods. Here, we report results of a 2-year farm-scale evaluation of the effects of transgenic cotton on biodiversity, pesticide use, and yield. We studied 81 commercial fields in a region of 6,600 km2 in Arizona, where Bt cotton represented 48% and 62% of the cotton planted in the first and second year of the study, respectively. Forty fields were planted to nontransgenic (nonTr) cotton, 21 fields to transgenic cotton producing the Bt toxin Cry1Ac (Bt), and 20 fields to cotton with Bt protein and herbicide resistance (BtHr). Bt cotton with Cry1Ac controls the pink bollworm (Pectinophora gossypiella), a major insect pest of cotton (9, 10).
Results and Discussion
Effects of Transgenic Cotton on Pesticide Use.
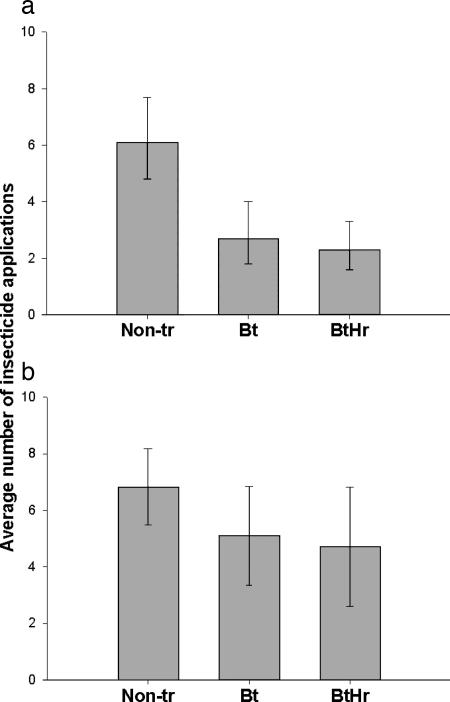
Transgenic cotton was treated with fewer broad-spectrum insecticides than nonTr cotton (Table 1, which is published as supporting information on the PNAS web site). In the first year of the study, the average number of insecticide applications in nonTr cotton was 6.6, which was significantly higher than in Bt (3.4) and BtHr (2.8) cotton (Fig. 1a). In the second year, the difference in insecticide use in nonTr cotton (6.8) compared with Bt (5.1) and BtHr (4.7) cotton was smaller but still significant (Fig. 1b). Insect growth regulators (IGRs), which are considered less harmful to nontarget arthropods than broad-spectrum insecticides, are used in Arizona for controlling the sweet potato whitefly (Bemisia tabaci) in cotton (11–14). Use of IGRs did not differ significantly between transgenic and nonTr cotton (Table 2, which is published as supporting information on the PNAS web site). The average number of herbicide applications, including or excluding glyphosate, did not differ significantly among nonTr, Bt, and BtHr cotton (Table 2 and Table 3, which is published as supporting information on the PNAS web site). Thus, lower use of broad-spectrum insecticides but similar use of herbicides occurred in transgenic cotton compared with nonTr cotton.
Fig. 1.
Average number of broad-spectrum insecticide applications in nonTr, Bt, and BtHr cotton (with 95% confidence intervals). The number of insecticide applications was significantly higher in nonTr than in transgenic cotton in 2002 (one-tailed contrast, t = 4.13, df = 72, P < 0.0001) (a) and in 2003 (one-tailed contrast, t = 1.99, df = 72, P = 0.025) (b). The number of insecticide applications was higher in 2003 than in 2002 (P = 0.058), although differences in insecticide applications among cotton types did not vary between years (P = 0.47).
Effects of Transgenic Cotton on Yield.
Average yield of cotton types (P = 0.17) and yield differences among cotton types (P = 0.33) did not vary between years. Least-squares means of yields for nonTr, Bt, and BtHr cotton were estimated with an analysis of covariance model, after controlling for the significant effects of insecticide use and seeding rate (see below). There was no significant difference between yields of Bt and BtHr cotton (P = 0.88). However, transgenic cotton produced more lint than nonTr cotton (one-tailed contrast, t = 1.87, df = 58, P = 0.033). The combined yield increase (least-squares mean ± SE) in Bt and BtHr cotton was 130.0 ± 69.5 kg·hectare (ha)−1, which represented 8.6% of the least-squares mean yield of nonTr cotton (1,509.0 ± 47.9 kg·ha−1).
Yield was positively associated with the number of broad-spectrum insecticide applications (log-transformed) (slope = 446.96, t = 3.24, df = 58, P = 0.002). However, no significant interaction occurred between cotton type, insecticide use, and year (P = 0.14) or between cotton type and insecticide use (P = 0.20), showing that yield of the cotton types was affected similarly by insecticide applications. Thus, yield of the cotton types increased at a slower rate with each additional insecticide application. Nevertheless, yield gain over the range of insecticides applied per field (0–16) was 550.0 kg·ha−1, 4.2 times greater than yield gain caused by use of transgenic cotton.
Seeding rate had a positive effect on yield (slope = 41.20, t = 2.45, df = 58, P = 0.017), but did not differ among nonTr, Bt, and BtHr cotton (P = 0.36). A yield gain of 288.4 kg·ha−1, twice the yield gain caused by use of transgenic cotton, occurred over the range of seeding rates (9.0–16.8 kg·ha−1).
The positive effect of broad-spectrum insecticides on yield demonstrates their key role in controlling major cotton pests. About 90% of broad-spectrum insecticides applied in Arizona cotton target the key pests B. tabaci, the western tarnished plant bug Lygus hesperus, and P. gossypiella (14). Bt cotton does not kill B. tabaci and L. hesperus. Thus, the rise in insecticide use in transgenic cotton in the second year of the study (Fig. 1) probably reflects an increasing need to control these two pests.
Because Bt cotton is resistant to P. gossypiella, transgenic cotton had higher yield than nonTr cotton for any given number of insecticide applications (see above). However, no overall yield difference occurred among nonTr, Bt, and BtHr cotton (P = 0.96). Based on the above analysis of covariance model, the higher use of insecticides in nonTr than in transgenic cotton (Fig. 1) increased yield by 137 and 51 kg·ha−1 in 2002 and 2003, respectively. Such yield gains compensated for gains caused by the use of transgenic cotton (i.e., 130.0 kg·ha−1). Thus, the similar yields in nonTr and transgenic cotton likely occurred because the additional insecticides applied in nonTr cotton significantly reduced damage caused by pests not killed by Bt cotton.
Effects of Transgenic Cotton on Biodiversity.
At least one edge of each cotton field was directly adjacent to noncultivated vegetation. To compare impacts of cultivation of nonTr and transgenic cotton on nontarget arthropods, we used pairwise comparisons of ant and beetle diversity in each type of cotton field with diversity of these taxa in adjacent noncultivated sites.
A total of 17,255 ants grouped in 9 morphospecies, 3 species groups, and 27 species were found in cotton fields and adjacent noncultivated sites. Species richness was higher in beetles than ants, as 10,444 beetles grouped in 23 morphospecies, 4 species groups, and 91 species were found at the same sites.
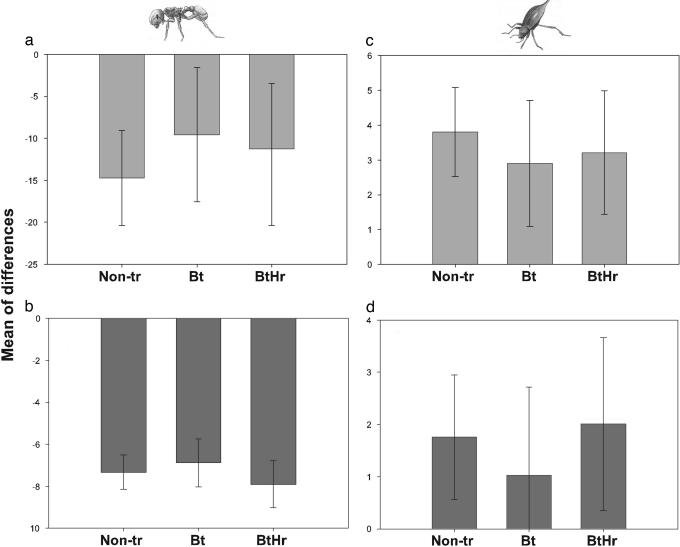
Ant density declined significantly from noncultivated vegetation to cotton fields (Fig. 2a). The average density decline was similar in nonTr, Bt, and BtHr cotton (P = 0.54). As expected (15), the density reduction in paired habitats was associated with a decline in ant species richness (one-tailed test; slope = 0.028, t = 1.70, df = 76, P = 0.047). However, the average reduction in species richness did not differ among cotton types (P = 0.44) (Fig. 2b). In contrast, beetle density increased from noncultivated vegetation to cotton fields (Fig. 2c). The average density increase did not differ among nonTr, Bt, and BtHr cotton (P = 0.69). Increased density in cotton fields was associated with a rise in beetle species richness (one-tailed test; slope = 0.33, t = 3.14, df = 76, P = 0.0012), whereas the average change in species richness did not differ among nonTr, Bt, and BtHr cotton (P = 0.69) (Fig. 2d). Thus, cultivation of transgenic and nonTr cotton had similar effects on diversity of nontarget insects.
Fig. 2.
Mean of differences (with 95% confidence intervals) in ant and beetle density or species richness between noncultivated vegetation and adjacent cotton fields, for nonTr, Bt, and BtHr cotton. (a and b) Ant density (a) and ant species richness (b) declined significantly from noncultivated vegetation to cotton fields. (c and d) Beetle density (c) and beetle species richness (d) increased significantly from noncultivated vegetation to cotton fields, except for species richness in Bt cotton. For ants, the year did not affect overall changes in density (P = 0.99) or species richness (P = 0.39) from noncultivated vegetation to the cotton types, nor the differences among changes in density (P = 0.98) or species richness (P = 0.13) in the cotton types. Similarly for beetles, the year did not affect overall changes in density (P = 0.85) or species richness (P = 0.73) from noncultivated vegetation to the cotton types, nor the differences among changes in density (0.64) or species richness (P = 0.96) in the cotton types.
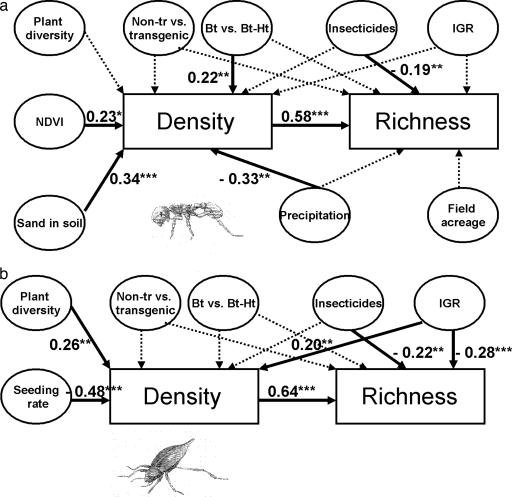
Several factors affecting ant and beetle diversity may have differed among nonTr, Bt, and BtHr cotton. Accordingly, the similar changes in ant and beetle diversity in the cotton types (Fig. 2) could have masked negative effects of transgenic cotton on these taxa. To assess this possibility, we used path analyses to evaluate the impacts of broad-spectrum insecticides, IGRs, transgenic cotton, and other factors (see Materials and Methods) on ant and beetle diversity in cotton fields. BtHr cotton reduced ant density compared with Bt cotton (Fig. 3a). However, a path analysis evaluating the effects of nonTr and BtHr cotton on ant density (results not presented) showed no significant difference between nonTr and BtHr cotton (P = 0.12). Furthermore, comparisons between nonTr and transgenic cotton did not reveal any significant impacts of transgenic cotton on ant diversity (Fig. 3a) and beetle diversity (Fig. 3b). In contrast, broad-spectrum insecticides significantly reduced ant and beetle species richness. Moreover, application of IGRs positively affected beetle density but negatively affected beetle species richness, resulting in an overall negative impact on beetle species richness (Fig. 3b).
Fig. 3.
Path analyses evaluating the effects of nonTr and transgenic cotton on density and species richness of ants and beetles (see Materials and Methods for details). Path coefficients not significantly different from zero are represented by dotted arrows; significant path coefficients are represented by continuous arrows. ∗, P < 0.07; ∗∗, P < 0.05; ∗∗∗, P < 0.01. (a) For ants, variables included in the analysis were the orthogonal contrast between nonTr and the weighted average of Bt and BtHr cotton (nonTr vs. transgenic), the orthogonal contrast between Bt and BtHr cotton (Bt vs. BtHr), number of broad-spectrum insecticide applications before insect sampling (insecticides), whether a field was treated with an IGR before insect sampling (IGR), number of plant types in noncultivated sites (plant diversity), NDVI of noncultivated sites, percentage of sand in soil (sand in soil), accumulated precipitation before insect sampling (precipitation), and field acreage. The compound path coefficients linking species richness to Bt vs. BtHr, NDVI, sand in soil, and precipitation were 0.13 (revealing lower species richness in BtHr than Bt cotton), 0.13, 0.20, and −0.19, respectively. (b) For beetles, the first five variables considered for ants and seeding rate were included in the path analysis. The compound path coefficients linking species richness to plant diversity and seeding rate were 0.17 and −0.31, respectively. The total correlation between IGRs and species richness was −0.15.
Characteristics of cotton fields (sand soil content and seeding rate) and noncultivated sites [Normalized Difference Vegetation Index (NDVI) and vegetation diversity] and accumulated precipitation significantly affected ant and beetle diversity (Fig. 3). Accumulated precipitation, NDVI, and seeding rate did not differ among cotton types (P > 0.31). Sand content (percentage ± SE) differed significantly among nonTr (44.0 ± 2.6), Bt (49.1 ± 3.6), and BtHr (55.5 ± 3.5) cotton [F(2,78) = 3.47, P = 0.036]. The average number of vegetation types (± SE) in noncultivated sites differed significantly among nonTr (3.22 ± 0.16), Bt (2.61 ± 0.22), and BtHr (2.50 ± 0.22) cotton [F(2,77) = 8.54, P = 0.016]. The significant differences in the number of vegetation types contributed in increasing beetle species richness in nonTr cotton (Fig. 3), thereby compensating for the negative impacts on beetle species richness of the higher use of broad-spectrum insecticides in nonTr cotton. However, as with the impacts of broad-spectrum insecticides, the significant difference in sand content among cotton types contributed in reducing ant species richness in nonTr cotton (Fig. 3), suggesting that factors not considered in this study were responsible for the similar ant diversity in nonTr and transgenic cotton (Fig. 2). Nevertheless, after controlling for the effects of several agronomic and ecological factors that significantly affected diversity of nontarget insects, there was still no evidence for different effects of nonTr and transgenic cotton on diversity of ants and beetles.
Our results demonstrate that use of transgenic cotton producing the toxin Cry1Ac in large commercial cotton fields reduced broad-spectrum insecticide use and increased yields at fixed insecticide levels. This increased yield benefits cotton producers because its value exceeds the additional cost of transgenic seeds. Overall, however, nonTr and transgenic cotton had similar yields. These results are consistent with other studies, mostly conducted in developed countries, in which pests targeted by Bt crops were fairly well controlled before deployment of Bt crops. In such cases, Bt crops generally substituted for insecticides without large yield improvements (4, 16–18). Constraints limiting efficacy of pest management are greater in developing countries where use of Bt crops appears more likely to substantially reduce insecticide use and improve yield (17–19). However, as shown here, replacement of broad-spectrum insecticides by narrow-spectrum Bt crops can reduce control of pests not affected by Bt crops. This finding suggests that the impacts of a particular Bt crop on agricultural productivity and insecticide use depend on whether insecticides are important for controlling pests not killed by Bt proteins in that crop. Thus, all key pests in a crop and their control methods should be considered in the decision to use Bt crops to mitigate the impacts of agricultural intensification.
Negative effects of cotton cultivation on ant diversity and positive effects on beetle diversity show that invertebrate taxa can react differently to agricultural perturbations. Ant and beetle diversity could have been affected negatively through direct exposure to Cry1Ac (e.g., after ingestion of plant residues, plant tissues, or prey containing a Bt toxin) or through changes in resources or shelters (e.g., lower abundance of prey or weeds) caused by the use of transgenic cotton (6, 16, 20). Nevertheless, greater impacts of transgenic cotton than nonTr cotton were not evident (Figs. 2 and 3). However, IGRs, broad-spectrum insecticides, and several other agronomic and ecological factors significantly affected ant and beetle diversity. Further experiments are needed to determine whether BtHr cotton has greater negative impacts on ant density than Bt cotton because of reduced biomass or diversity of weeds (6, 21). Recently published experimental (22–25) and large-scale (26) studies confirm that impacts on insect communities are much greater for broad-spectrum insecticides than for Bt crops.
Generalizations about environmental impacts of transgenic crops are difficult because farmer management practices influence such impacts and constraints on management practices vary regionally (6, 16–18). Nevertheless, our findings indicate that Bt crops could be useful to reduce environmental impacts of agricultural intensification, especially where replacement of insecticides by Bt crops will not reduce the control of pests unaffected by Bt proteins.
Materials and Methods
Sampling Protocol.
To select experimental fields in western Pinal County, Arizona, we used Geographical Information System (GIS) maps that identified with high accuracy the location of cotton fields and the type of cotton planted in each field (27). Fields were arbitrarily selected within a region of ≈6,600 km2, which was delimited by the frames of Landsat-7 Enhanced Thematic Mapper Plus satellite images overlaid on the GIS maps. For each year of the study, the percentage of Bt cotton in the region was determined from analyses of the GIS maps (27).
Each selected field had at least one border adjacent to noncultivated vegetation. The location and shape of fields, as well as the position of pitfall traps placed inside and outside the fields (see below), were mapped with a Global Positioning System. Selected fields were different in 2002 and 2003.
Sampling was conducted during the monsoon season (August to mid-September), which corresponds to the peak of arthropod diversity in the Sonoran desert (28). Two transects, 100 m apart, were established with pitfall traps at each site. Transects started 140 m inside a cotton field and extended 140 m inside the paired noncultivated site. Pitfall traps were 20 m apart, for a total of 14 pitfall traps placed in each cotton field and 14 traps placed in each noncultivated site. Pitfall traps were left in the ground for 48 h, after which they were brought to the laboratory for specimen identification.
Specimen Identification.
Ant winged males and queens were not included in analyses. Ants and beetles were pinned and identified to species with existing keys. Specimens that could not be matched to species were assigned to morphospecies. In some cases, individuals from two very similar species were not separated because of logistical constraints and were assigned to the same species group. Vouchers are deposited in the entomological collection of the Department of Entomology at the University of Arizona. Vouchers of two Temnothorax ant species identified in this study are deposited at the Museum of Comparative Zoology, Harvard University, Boston.
Site Characterization.
Cotton producers participating in the study completed questionnaires from which information on the following variables was obtained: cotton type planted (cultivar brand); distance between cotton rows (in centimeters); harvest date; number and date of irrigations (all farmers used flood irrigation) and tillages; number, date, and type of product used in applications of broad-spectrum insecticides, herbicides, and IGRs (pyriproxyfen and buprofezin); planting date; seeding rate (kg·ha−1); seed depth (in centimeters); and yield as recorded at the gin (bales or lb·acre−1). Yield was converted to kg·ha−1 for statistical analyses, assuming that a cotton bale weighed 226.8 kg.
In each cotton field, soil samples were taken at ≈1 m from seven randomly selected pitfall traps. The seven samples were pooled and brought to the Soil, Water, and Plant Analysis Laboratory at the University of Arizona, where soil texture (i.e., percentage sand, silt and clay), total nitrogen and carbon content, and soil pH were determined.
Meteorological stations across the study area (29) were used to estimate the accumulated precipitation (in centimeters) from cotton planting to harvest, and from cotton planting to initiation of insect sampling, for each field. Heat unit accumulation from planting to harvest was estimated with the single sine curve method, using thresholds of cotton development of 30.00/12.78°C (30). Global Positioning System coordinates were used to measure field altitude (in meters) and calculate the area (in square meters) and edge (i.e., perimeter divided by area, m−1) of each field.
Landsat-7 Enhanced Thematic Mapper Plus satellite images were used to measure NDVI in each noncultivated site. NDVI values reflect biomass and chlorophyll content of green vegetation and provide a landscape-scale index of the “greenness” of a patch (31). NDVI values were measured in an area of 300 × 240 m, which was directly adjacent to, but not overlapping, the sampled cotton fields, and was centered on the transects of pitfall traps in noncultivated sites. In 2002, NDVI values were derived from images taken on June 5, July 7, and August 24. Images obtained on May 15, July 2, and August 3 were analyzed in 2003. For each year and noncultivated site, the NDVI values from the three images were used to calculate an average NDVI and the associated coefficient of variation.
Each noncultivated site was classified according to the number of “vegetation types” present. Presence/absence of nine types were recorded: cacti, creosote bush (Larrea tridentata), flowering weeds, grasses, mesquite (Prosopis velutina), paloverde (Cercidium floridum), salt cedar (Tamarix ramosissima), rabbit brush (Chrysothamnus nauseosus), and “other brush.”
Statistical Analyses.
Some cotton producers did not report data for all variables, which resulted in an unequal number of observations for variables included in statistical analyses. Two-way ANOVA was used to evaluate the effects of year, cotton type (nonTr, Bt, and BtHr), and their interaction on the number of broad-spectrum insecticide applications [log(X + 1)-transformed] or herbicide applications in cotton fields. Contrasts were used to further assess differences among cotton types in the average number of insecticide applications. Logistic regression was first used to evaluate the effect of year, cotton type (Bt and BtHr), and their interaction on the odds of an IGR application in cotton fields. Logistic regression was then used to compare IGR applications between nonTr and transgenic cotton.
Stepwise regression was first used to identify the factors affecting yield. Factors included in the stepwise regression procedure were heat unit accumulation from planting to harvest, length of growing season, number of herbicide applications, number of broad-spectrum insecticide applications [log(X + 1)-transformed], number of irrigations, percentage of sand, silt, or clay in soil, row spacing, seeding rate, seed depth, soil pH, soil nitrogen or carbon content (both log-transformed), and whether a field was treated with an IGR. In a second stage, significant factors (P < 0.05) retained in the stepwise regression procedure were included as covariates in an analysis of covariance model, which assessed the effect of year, cotton type, number of broad-spectrum insecticide applications, seeding rate, and their interactions.
Two-way ANOVA was used to assess the effects of year, cotton type, and their interaction on the difference in ant or beetle density (average number of insects per pitfall trap) between the paired noncultivated sites and cotton fields. Analysis of covariance was used to evaluate the effects of year, cotton type, the difference in ant or beetle density between paired sites, and the interaction between year and cotton type, on the difference between the number of ant or beetle morphospecies, species groups, and species found at the paired sites.
We used path analyses (32, 33) to assess the effects of transgenic cotton on ant and beetle diversity. Before performing the path analyses, stepwise regression was used to identify the variables potentially affecting ant and beetle density or species richness. Variables included in the stepwise regression procedure were altitude of field, accumulated precipitation from crop planting to sampling of insects, average and coefficient of variation of NDVI values of noncultivated sites, edge of field, field area (log-transformed), number of herbicide applications before insect sampling, number of irrigations before insect sampling, number of plant types in noncultivated sites, percentage of sand, silt, or clay in soil, plant seeding rate, row spacing, soil pH, soil nitrogen and carbon content (both log-transformed), and year. Variables retained in the stepwise procedure (P < 0.05) were added to variables describing insecticide use in the cotton fields for the path analyses. Two orthogonal contrasts, taking into account the unequal sample sizes for the types of fields, were used to assess the potential effects of transgenic cotton. The first contrast compared nonTr cotton with a weighted average of Bt and BtHr cotton, whereas the second compared Bt with BtHr cotton. The other variables describing insecticide use were the number of broad-spectrum insecticides applied, and whether a field was treated with an IGR, before insect sampling.
Two-way ANOVA was used to assess the effects of year, cotton type, and their interaction on accumulated precipitation, NDVI, number of plant types in noncultivated sites, seeding rate, and percentage of sand in soil.
Supplementary Material
Acknowledgments
A. Ali, K. Dell, G. Fugate, J. Harms, and D. Overton provided field and laboratory assistance. S. Cover, J. Ellington, and D. Wheeler helped with specimen identification. M. Sisterson, B. Tabashnik, and K. Walker provided comments on the manuscript. Environmental Protection Agency Cooperative Agreement X-82974701-O provided support for this study.
Abbreviations
- Bt
Bacillus thuringiensis
- BtHr
Bt protein and herbicide resistance
- nonTr
nontransgenic
- IGR
insect growth regulator
- ha
hectare
- NDVI
Normalized Difference Vegetation Index.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tilman D., Fargione J., Wolff B., D’Antonio C., Dobson A., Howarth R., Schindler D., Schlesinger W. H., Simberloff D., Swackhamer D. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 2.Khush G. S. Nat. Rev. Genet. 2001;2:815–822. doi: 10.1038/35093585. [DOI] [PubMed] [Google Scholar]
- 3.Pimentel D. S., Raven P. H. Proc. Natl. Acad. Sci. USA. 2000;97:8198–8199. doi: 10.1073/pnas.97.15.8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J., Rozelle S., Pray C., Wang Q. Science. 2002;295:674–677. doi: 10.1126/science.1067226. [DOI] [PubMed] [Google Scholar]
- 5.Hails R. S. Nature. 2002;418:685–688. doi: 10.1038/nature01016. [DOI] [PubMed] [Google Scholar]
- 6.Squire G. R., Brooks D. R., Bohan D. A., Champion G. T., Daniels R. E., Haughton A. J., Hawes C., Heard M. S., Hill M. O., May M. J., et al. Philos. Trans. R. Soc. London B. 2003;358:1779–1799. doi: 10.1098/rstb.2003.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Callaghan M., Glare T. R., Burgess E. P. J., Malone L. A. Annu. Rev. Entomol. 2005;50:271–292. doi: 10.1146/annurev.ento.50.071803.130352. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence S. Nat. Biotechnol. 2005;23:281. doi: 10.1038/nbt0305-281. [DOI] [PubMed] [Google Scholar]
- 9.Tabashnik B. E., Patin A. L., Dennehy T. J., Liu Y.-B., Carrière Y., Sims M. A., Antilla L. Proc. Natl. Acad. Sci. USA. 2000;97:12980–12984. doi: 10.1073/pnas.97.24.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrière Y., Ellers-Kirk C., Sisterson M., Antilla L., Whitlow M., Dennehy T. J., Tabashnik B. E. Proc. Natl. Acad. Sci. USA. 2003;100:1519–1523. doi: 10.1073/pnas.0436708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennehy T. J., Williams L. Pest. Sci. 1997;51:398–406. [Google Scholar]
- 12.Ellsworth P. C., Martinez-Carillo J. L. Crop Protec. 2001;20:853–869. [Google Scholar]
- 13.Naranjo S. E., Ellsworth P. C., Hagler J. R. Biol. Contr. 2004;30:52–72. [Google Scholar]
- 14.Carrière Y., Sisterson M. S., Tabashnik B. E. In: Insect Pest Management: Field and Protected Crops. Horowitz A. R., Ishaaya I., editors. New York: Springer; 2004. pp. 65–95. [Google Scholar]
- 15.Gotelli N. J., Colwell R. K. Ecol. Lett. 2001;4:379–391. [Google Scholar]
- 16.Carpenter J., Felsot A., Goode T., Hammig M., Onstad D., Sankula S. Comparative Environmental Impacts of Biotechnology-Derived and Traditional Soybean, Corn, and Cotton Crops. Ames, IA: Council for Agricultural Science and Technology; 2002. [Google Scholar]
- 17.Quaim M., Ziberman D. Science. 2003;299:900–902. doi: 10.1126/science.1080609. [DOI] [PubMed] [Google Scholar]
- 18.Fitt G. P., Wakelyn P. J., Stewart J., James C., Roupakias D., Hake K., Zafar Y., Pages J., Giband M. Global Status and Impacts of Biotech Cotton: Report of the Second Expert Panel on Biotechnology of Cotton. Washington, DC: International Cotton Advisory Committee; 2004. [Google Scholar]
- 19.Huang J., Hu R., Rozelle S., Pray C. Science. 2005;308:688–690. doi: 10.1126/science.1108972. [DOI] [PubMed] [Google Scholar]
- 20.Zwahlen C., Andow D. A. Environ. Biosafety Res. 2005;4:113–117. doi: 10.1051/ebr:2005014. [DOI] [PubMed] [Google Scholar]
- 21.Brooks D. R., Bohan D. A., Champion G. T., Haughton A. J., Hawes C., Heard M. S., Clark S. J., Dewar A. M., Firbank L. G., Perry J. N., et al. Philos. Trans. R. Soc. London B. 2003;358:1847–1862. doi: 10.1098/rstb.2003.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dively G. P. Environ. Entomol. 2005;34:1267–1291. [Google Scholar]
- 23.Naranjo S. E. Environ. Entomol. 2005;34:1193–1210. [Google Scholar]
- 24.Naranjo S. E. Environ. Entomol. 2005;34:1211–1223. [Google Scholar]
- 25.Whitehouse M. E. A., Wilson L. J., Fitt G. P. Environ. Entomol. 2005;34:1224–1241. [Google Scholar]
- 26.Head G., Moar W., Eubanks M., Freeman B., Ruberson J., Hagerty A., Turnipseed S. Environ. Entomol. 2005;34:1257–1266. [Google Scholar]
- 27.Carrière Y., Ellers-Kirk C., Kumar K., Heuberger S., Whitlow M., Antilla L., Dennehy T. J., Tabashnik B. E. Pest Manag. Sci. 2005;61:327–330. doi: 10.1002/ps.1039. [DOI] [PubMed] [Google Scholar]
- 28.Schumacher A., Whitford W. G. Southwest. Nat. 1976;21:1–8. [Google Scholar]
- 29.Brown P. W., Russell B. Weather Data and On-Line Documentation. Tucson: Arizona Meteorological Network; 1995. [Google Scholar]
- 30.Brown P. W., Silvertooth J. F., Watson T. F. In: Cotton: A College of Agriculture Report. Silvertooth J. F., editor. Tucson: University of Arizona College of Agriculture and Life Sciences; 1992. pp. 421–451. [Google Scholar]
- 31.Jensen J. R. Remote Sensing of the Environment: An Earth Resource Perspective. Upper Saddle River, NJ: Prentice–Hall; 2000. [Google Scholar]
- 32.Vaudor A. piste. Montreal: Université de Montréal; 1991. Version 3.0. [Google Scholar]
- 33.Sokal R. R., Rohlf F. J. Biometry: The Principles and Practice of Statistics in Biological Research. New York: Freeman; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.