Abstract
Vasopressin neurons in the bed nucleus of the stria terminalis and amygdala and vasotocin neurons in homologous areas in non-mammalian vertebrates show some of the most consistently found neural sex differences, with males having more cells and denser projections than females. These projections have been implicated in social and reproductive behaviors but also in autonomic functions. The sex differences in these projections may cause as well as prevent sex differences in these functions. This paper discusses the anatomy, steroid dependency, and sexual differentiation of these neurons. Although the final steps in sexual differentiation of vasopressin/vasotocin expression may be similar across vertebrate species, what triggers differentiation may vary dramatically. For example, during development, estrogen masculinizes vasopressin expression in rats but feminizes its counterpart in Japanese quail. Apparently, nature consistently finds a way of maintaining sex differences in vasopressin and vasotocin pathways, suggesting that the function of these differences is important enough that it was conserved during evolution.
Keywords: sex differences, testosterone, estrogen, bed nucleus of the stria terminalis, amygdala, lateral septum
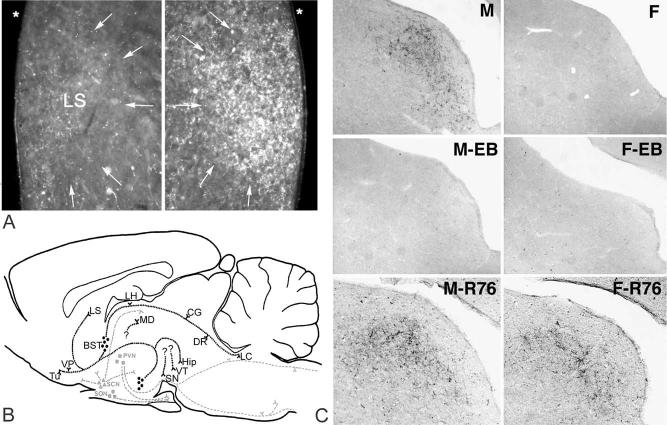
Sex differences in vasopressin (AVP) projections of the bed nucleus of the stria terminalis (BST) and medial amygdaloid nucleus (MeA) were first described in rats and were discovered by chance while we were studying what we thought were developing AVP projections of the suprachiasmatic nucleus (SCN) (De Vries et al., 1981). AVP cell bodies in the BST and MeA had yet to be discovered (Van Leeuwen and Caffé, 1983). A study of the development of vasopressin-immunoreactive (AVP-ir) fibers in the lateral septum and habenular nucleus revealed a disturbingly large variability, prompting us to run a second series, separating animals by sex. This revealed a large sex difference with males having a much denser AVP-ir fiber network in the lateral septum and lateral habenular nucleus than females (Fig. 1A). Later we showed that AVP expression in these areas critically depended on circulating gonadal steroids (De Vries et al., 1984), and that AVP-ir cells in the BST and MA showed corresponding sex differences and steroid responsiveness (DeVries et al., 1985; Van Leeuwen et al., 1985). Since these first findings, homologous sex differences have been found in many different species, in mammals as well as other vertebrates (Table 1).
Fig. 1.
Sexually dimorphic AVP and vasotocin (AVT) projections in rat and quail brains. (A) Dark-field microphotographs of AVP-ir fiber networks (arrows) in the lateral septum (LS) of a female (left) and male rat (right); * lateral ventricle. (B) Diagram of most prominent AVP-ir projections in rats, modified from De Vries et al. (1985). Steroid-sensitive projections (black lines) run from BST (circles) and MeA (MA, circles) to LS, ventral pallidum (VP), olfactory tubercle (Tu), lateral habenular nucleus (LH), midbrain central gray (CG), dorsal raphe nucleus (DR), locus coeruleus (LC), and ventral hippocampus (Hip). Question marks indicate projections to Hip, mediodorsal nucleus of the thalamus (MD), ventral tegmental area (VT), substantia nigra (SN), which disappeared after castration but not after lesioning the BST. Steroid-insensitive projections (gray lines) originate in SCN (triangles), PVN (squares), and supraoptic nucleus (SON, squares). (C) Bright-field photomicrographs of AVT-ir fiber networks in the lateral septum of male (M) and female Japanese quail (F), treated during development with oil (top panels), estradiol benzoate (EB; middle panels), or the aromatase inhibitor R76 (R76; bottom panels), gonadectomized three weeks post-hatching, and treated with testosterone for another two weeks. Note that AVT fibers are absent in the oil-treated female and EB-treated male and female quail.
Table 1.
Studies on sex differences and steroid effects in AVP and AVT expression in BST / MeA and homologous systems
| Projections | Cell bodies | Dimorphism | Seasonal | Activational | A vs. E | Organizational | Tracing | |
|---|---|---|---|---|---|---|---|---|
| Mammals | ||||||||
| Rat, R. rattus | 1, 8 | 3, 8, 6, 21 | 2, 10, 25 | 2, 5, 43 | 7, 8, 26, 37 | 12, 42, 48, 56 | 27, 45, 48 | 4, 14 |
| Mouse, M. musculus | 19 | 19 | 20 | — | 20 | 20, 80, 86 | — | — |
| Siberian hamster, P. sungorus | 49 | 58, 49 | 49* | 58 | 49 | — | 58 | — |
| Prairie vole, M. ochrogaster | 41, 51 | 41, 51 | 41, 51 | — | 44 | 72 | 88 | — |
| Mongolian gerbil, M. unguiculatus | 34 | 34 | 34 | — | 34 | — | 34 | — |
| Gerboa, J. orientalis | 55 | 55 | 55 | 55 | — | — | — | — |
| Meadow vole, M. pennsylvanicus | 41, 51 | 41, 51 | 41, 51 | — | — | — | — | — |
| Dormouse, E. quercinus | 28 | — | 28 | 28 | — | — | — | — |
| European hamster, C. cricetus | 11 | — | 11 | 11 | — | — | — | — |
| Guinca pig, C. porcellus | 24 | 24 | 24* | — | — | — | — | — |
| Human, H. sapitens | 13 | 13 | 13* | — | — | — | — | — |
| Macaque, M. fascicularis | 23 | 23 | 23* | — | — | — | — | — |
| Marmoset, C. jacchus | 65* | 65 | 65 | — | — | — | — | — |
| Montane vole, M. montanus | 61 | 61 | 61 | — | — | — | — | — |
| Pine vole, M. pinetorum | 61 | 61 | 61 | — | — | — | — | — |
| Brazilian opossum, M. domestica | 54 | — | 54 | — | — | — | — | — |
| Cat, F. catus | — | 15 | — | — | — | — | — | — |
| California mice, P. californicus | 69 | 69 | — | — | — | — | — | — |
| Mustached bat, P. parnellii | 85 | 85 | — | — | — | — | — | — |
| Syrian hamster, M. auratus | 30* | 30* | — | — | — | — | — | — |
| White-footed mice, P. leucopus | 69 | 69 | — | — | — | — | — | — |
| Birds | ||||||||
| Japanese quail, C. Japonica | 39, 50, 77 | 66, 66, 77 | 39, 66, 66, 77 | — | 50, 74, 60 | 78 | 68, 90 | 79 |
| Canary, S. canaria | 17 | 17 | 22 | 31 | — | 22* | — | |
| Zebra finch, T. guttata | 40 | 40 | 71, 40* | 40* | 40* | — | — | — |
| Chicken, G. domesticus | 64 | 57/64 | 64 | 70 | — | — | — | — |
| Junco, J. hyemalis | 75 | 75 | — | — | 84 | — | — | — |
| White-throated sparrow, Z. albicollis | 89 | 89 | 89 | — | — | — | — | — |
| Budgerigar, M. undulates | 82* | 82* | — | — | — | — | — | — |
| Angolan blue waxbill, U. angolensis | 83 | — | — | — | — | — | — | — |
| Song sparrow, M. melodia | 83 | — | — | — | — | — | — | — |
| Spice finch, L. punctulata | 83 | — | — | — | — | — | — | — |
| Violet-eared waxbill, U. granatina | 83 | — | — | — | — | — | — | — |
| Reptiles | ||||||||
| Ball python, P. regius | 29 | 29 | 29 | — | — | — | — | — |
| Gecko, G. gecko | 9, 18 | 9, 18 | 9, 18 | — | — | — | — | — |
| Green anole, A. carolinensis | 38 | 38 | 38 | — | — | — | — | — |
| Red-eared slider, P. scripta | 29 | 29 | 29 | — | — | — | — | — |
| Jararaca pitviper, B. jararaca | 81 | 81 | — | — | — | — | — | — |
| Chameleon, C. chamaeleon | 52* | 52* | — | — | — | — | — | — |
| Amphibia | ||||||||
| Bullfrog, R. catesbaiana | 33 | 33 | 32, 33 | 46 | 47 | 47 | 47 | — |
| Roughskin newt, T. granulosa | 63 | 63, 87 | 32, 76 | 76* | — | — | — | — |
| Cricket frog, A. crepitans | 73 | 73 | — | — | — | — | — | |
| South African clawed frog, X. laevis | 35 | 35 | — | 53 | — | — | — | — |
| Wood frog, R. sylvatica | 59 | 59 | — | 59 | — | — | — | — |
| Marsh frog, R. ridibunda | 36 | 36 | — | — | — | — | — | — |
| Rubber eel, T. compressicauda | 62 | 62 | — | — | — | — | — | — |
| Rubber eel, T. natans | 67 | 67 | — | — | — | — | — | — |
| Spanish ribbed newt, P. waltlii | 36 | 36 | — | — | — | — | — | — |
| Japanese toad, B. japonicus | 16 | — | — | — | — | — | — | — |
| Red-legged salamander, P. shermani | — | 87 | — | — | — | — | — | — |
Studies on AVP/AVT expression in BST, MeA, and homologous systems. Numbers match chronology. Species are grouped according to amount of data generated and then alphabetically. ‘Projections’ and ‘Cell bodies’: AVP/AVT peptide or mRNA (numbers in italics) in projections or cell bodies, respectively; ‘Dimorphism’: sex differences in expression; ‘Seasonal’: variability likely caused by seasonal or developmental variability in steroids; ‘Activational’: effects of circulating steroids: ‘A vs. E’: estrogenic vs. androgenic effects; ‘Organizational’: developmental effects of steroids or persistence of differences after equal steroid treatment; ‘Tracing’: origin of AVP/AVT projections; ‘-’: feature not studied;
feature not found.
24 Dubois-Dauphin et al., 1989
39 Viglietti-Panzica et al., 1992
46 Boyd, 1994a
47 Boyd, 1994b
49 Dubois-Dauphin et al., 1994
50 Viglietti-Panzien et al., 1994
55 Lakhdar-Ghazal et al., 1995
67 Hilscher-Conklin et al., 1998
69 Bester-Meredith et al., 1999
78 Viglietti-Panzica et al., 2001
Sources of sexually dimorphic AVP and vasotocin innervation
In rats, the BST and MeA provide a major part of AVP innervation in the forebrain. These two areas belong to the extended amygdala, a cohort of nuclei in the BST and centromedial amygdala with striking similarities in cytoarchitecture and neurochemistry (de Olmos and Heimer, 1999). The sexual dimorphism and steroid responsiveness of AVP neurons in BST as well as MeA underscore these similarities. In vertebrates with less extensive encephalization than mammals, the areas homologous to the BST and amygdala are not physically separated by the internal capsule (Johnston, 1923). In such animals, the sexually dimorphic vasotocin-immunoreactive (AVT-ir) cells typically form a single, undivided cluster (e.g. Boyd et al., 1992; Marler et al., 1999).
Given the wide acceptance of our proposal that the BST and MeA are the sources of sexually dimorphic AVP-ir and, by extension, AVT-ir innervation of the brain, it is important to point out that this idea is based on a rather limited set of experiments (De Vries and Buijs, 1983). To locate the source of the sexually dimorphic AVP-ir inner-vation of the lateral septum, we used knife cuts ventral to the septum, which showed that AVP-ir fibers enter the septum ventrorostrally. We retrogradely traced connections to the lateral septum (regardless of neuropeptide expression), which favored the BST as a source over other likely candidates, i.e. the SCN and paraventricular nucleus (PVN). Finally, because lesions of the SCN had already disqualified the SCN as a likely source of septal AVP-ir innervation (Hoorneman and Buijs, 1982), we lesioned the PVN bilaterally, which spared septal AVP-ir innervation, and the BST unilaterally, which decimated septal AVP-ir innervation ipsilaterally (bilateral lesions caused high mortality). The conclusion that the BST is an important source of septal AVP-ir fibers therefore rests on solid evidence (De Vries and Buijs, 1983). Evidence for other projections from the BST and MeA is less firm. When we found that castration deleted AVP-ir cell bodies in the BST and MeA but not in other areas, and AVP-ir fibers in all areas where unilateral lesions of the BST had eliminated AVP-ir fibers, we proposed that the BST and MeA projects to all areas where castration eliminated AVP immunoreactivity and not to areas where AVP immunoreactivity remained (Fig. 1B; DeVries et al., 1985). Later Caffé et al. (1987) combined retrograde tracing with AVP immunocytochemistry to confirm that the BST projects to the lateral septum and show that the MeA projects to the ventral hippocampus as well as lateral septum. Differences in the effects of BST and MeA lesions on septal AVP innervation, however, suggest that the BST provides the lion's share of the septal AVP-ir innervation (Al Shamma and De Vries, unpublished observations). None of the other BST and MeA projections have been independently confirmed. Even less certainty exists about homologous projections in other vertebrates. The only other tracing study was done in Japanese quail, which demonstrated that AVT-ir neurons in the BST project to the medial preoptic nucleus (POM; Absil et al., 2002). However, given the often striking similarity in distribution, sexual dimorphism, and steroid sensitivity of AVP and AVT systems across vertebrates, it is unlikely that the anatomy of these systems differs fundamentally among species.
There are some intriguing species differences, however. For example, Moore et al. (2000) find similar sex differences in roughskin newts as are found in rats. However, they also find more AVT-expressing cell groups than have been found in any other species. Some of these cell groups exhibit differences favoring males, others females. Any number of these cell groups could contribute to sexually dimorphic AVT-ir innervation. One could even imagine that some of these cell groups cancel out differences in fiber density if cell groups with opposite differences project to overlapping areas. Like roughskin newts, Japanese quail show similar differences in the BST as do rats, but male quail also have more AVT-ir cell bodies in the POM than do females (Panzica et al., 2001). As the POM projects to the lateral septum (Balthazart et al., 1994), these cell bodies may contribute to differences in septal AVT-ir innervation as well. Sex differences have also been found in AVP/AVT neurons in the PVN in mammals, including humans (Ishunina and Swaab, 1999), and the preoptic area in non-mammalian vertebrates, especially in fish (e.g. Grober and Sunobe, 1996; Semsar and Godwin, 2003). Many of these neurons appear neurosecretory, however, and may therefore not contribute to central AVP/AVT-ir innervation.
AVP-ir fibers in the lateral septum are sometimes used as a yardstick for the AVP projections from the BST and MeA. For example, macaques, marmosets, and humans show virtually no AVP-ir fibers in the lateral septum and therefore no clear sex difference in this area either. These primates, however, have AVP-ir cells in the BST, and male marmosets have more of these cells than do females (Caffé et al., 1989; Fliers et al., 1986; Wang et al., 1997). In fact, primate and rodent AVP innervation may be quite similar, because many areas that receive steroid-sensitive AVP innervation in rats, such as the ventral pallidum, dorsal raphe nucleus, and midbrain central gray, also contain dense AVP-ir inner-vation in macaques (Caffé et al., 1989). As human brains have not been studied as comprehensively, sex differences in AVP projections in the human brain cannot be discounted.
There are major outliers, however. For example, Syrian hamsters lack homologous steroid-sensitive AVP cells in the BST and MeA and AVP innervation in areas such as the lateral septum that would have received innervation from those cells (Albers et al., 1991; Ferris et al., 1995; Miller et al., 1999). Within mammals, Syrian hamsters are exceptional, however. Given that homologous AVT cells have never been reported for fish either (Goodson and Bass, 2001), fish may be the only vertebrate class that does not have the steroid-sensitive systems reviewed in this paper. However, these systems may have escaped detection; AVP cells were discovered in BST and MeA only after colchicine treatment (Van Leeuwen and Caffé, 1983), and visualizing them without colchicine required increasing the sensitivity of immunocytochemistry and in situ hybridization (De Vries et al., 1985; Miller et al., 1988).
Hormonal and genetic factors in sexual differentiation of AVP/AVT pathways
The development of sex differences in the steroid-sensitive AVP/AVT projections have been studied in a limited number of species only (Table 1). Here, we will review rats, the animals most extensively studied, contrasting them with other species in case fundamental differences have been found.
In mammals, the main factors in sexual differentiation of the brain are gonadal hormone levels during development and in adulthood (Becker et al., 2005). Gonadal hormones influence sexual differentiation in at least two ways. Early in life, they permanently direct the development of neural circuitry that will generate male- or female-typical functions and behaviors in adulthood. These developmental effects are called organizational. For example, testosterone exposure during development increases the likelihood that animals will show male sexual behavior as adults. However, to show male sexual behavior, animals have to be exposed to testosterone in adulthood as well. This adult effect is transient and therefore called activational. Sex differences in AVP/AVT innervation of the brain depend on organizational and activational effects of gonadal hormones and possibly also directly on sex chromosomal complement.
In adulthood, AVP projections from the BST and MeA are exquisitely sensitive to circulating gonadal hormones. Gonadectomy eliminates AVP expression and replacement of hormones reinstates it (e.g. De Vries et al., 1984; Miller et al., 1992). Sex differences in circulating gonadal hormones, however, cannot explain all differences in AVP expression because males and females exposed to similar steroid levels still differ (De Vries and al Shamma, 1990; De Vries et al., 1994). Circulating hormones may, however, be the main factor in species such as prairie voles, where differences in AVP-ir fiber density (Bamshad et al., 1993) or AVP mRNA expression (Wang et al., 1994) are extreme. These differences are still impressive, but smaller if adult male and female voles are treated with similar levels of testosterone (Lonstein et al., 2005). Like prairie voles, Japanese quail and chickens show extreme sex differences with females showing virtually no AVT-immunoreactivity in the BST and its projections, and barely any AVT mRNA signal (Viglietti-Panzica et al., 1994; Jurkevich et al., 1997; Aste et al., 1998). These differences remain extreme in quail treated similarly with testosterone (Fig. 1C; Panzica et al., 1998). In rats as well as quail, such residual differences are due to organizational effects of hormones (Wang et al., 1993; Panzica et al., 1998; Han and De Vries, 2003).
In mice, sex chromosomal complement (XX versus XY in mammals and ZW versus ZZ in birds) may bias form and function of neural systems independently of its effects on gonadal differentiation (Arnold, 2004). We showed that this is true for AVP innervation by using a cross in which sex chromosomal complement was varied independently of gonadal sex. This cross generated XX and XY mice lacking the Sry gene on the Y chromosome, which normally directs testis growth; these mice developed a female phenotype. This cross also generated XX and XY animals with an Sry transgene on an autosomal chromosome; these mice developed a male phenotype. Whether male or female, XY mice had a slightly denser AVP innervation than did XX mice even though gonadal hormone levels were kept constant (De Vries et al., 2002).
Mechanisms that trigger sexual differentiation of AVP/AVT systems may differ markedly among vertebrates. For example, in voles, testes are essential for masculinization of AVP innervation. However, administering testosterone to females or to neonatally castrated males does not masculinize this system, raising doubt that the testis uses testosterone to masculinize AVP expression (Lonstein et al., 2005). Even more striking differences are found between rats and Japanese quail. In both species, testosterone activates AVP/AVT expression mainly by acting via estrogenic metabolites (De Vries et al., 1986, 1994; Viglietti-Panzica et al., 2001). Notwithstanding this and other striking similarities in steroid responsiveness in adulthood (Panzica et al., 2001), hormones act in opposite directions during sexual differentiation. In rats, estrogen masculinizes AVP innervation (Han and De Vries, 2003); in quail, estrogen feminizes its AVT counterpart (Fig. 1C; Panzica et al., 1998). Even though the triggers of sexual differentiation may differ, the consistency of sex differences in AVP and AVT systems suggests that cellular processes that shape these differences may be similar among vertebrates.
Cellular processes behind sexual differentiation of AVP /AVT pathways
Differences in number of AVP and AVT cells may account for differences in fiber density. In theory, two fundamentally different sets of processes could determine AVP/AVT cell number: processes that determine absolute cell number, such as cell birth, cell death, or cell migration, or processes that influence the phenotype of existing cells. Differential cell birth and migration are unlikely players because AVP cells are born on embryonic days 12 and 13 (al Shamma and De Vries, 1996), at least a week before hormones trigger their sexual differentiation (Wang et al., 1993). Differential cell death is also unlikely. Recently, we compared wild-type mice and mice with a null mutation in the gene coding for the cell death factor, Bax. This mutation thwarts most neuronal cell death and eliminates sex differences in cell number of several brain areas (Forger et al., 2004) but not sex differences in AVP cell number (De Vries, Reza, and Forger, unpublished observations). It did increase AVP cell number in both sexes, though, suggesting that developmentally programmed cell death determines the final number of potential AVP cells in males and females but not the differences between them.
All evidence points at testosterone stimulating already existing cells to express AVP. After discovering that practically all AVP cells in the BST co-express galanin, but not all galanin cells AVP, and that males have more AVP cells than do females but similar numbers of galanin cells, Planas et al. (1995) proposed that differences in AVP expression depend on the percentage of galanin cells that co-express AVP. During development, testosterone may simply stimulate more galanin neurons to co-express AVP in males. In support, AVP and galanin neurons in the BST and MeA show an equally unusual birth profile: both are born days earlier than most surrounding cells (Han and De Vries, 1999). A similar situation may apply in Japanese quail, where AVT-ir and galanin-ir cells overlap in the BST (Aste et al., 1996; Azumaya and Tsutsui, 1996), and in songbirds, where AVT-ir and galanin-ir fibers overlap in the lateral septum (Goodson et al., 2004).
It is unknown whether gonadal steroids target AVP and AVT cells directly or act via other cells. In adult rats, steroid actions may be direct because AVP-ir cells in the BST and MeA express estrogen receptor alpha, androgen, and progesterone receptors (Axelson et al., 1992; Zhou et al., 1994; Auger and De Vries, 2002). It is unknown whether these cells also express gonadal steroid receptors during sexual differentiation. Finally, no study has addressed co-localization of AVP and AVT steroid receptors and these cells in other vertebrates.
A unique opportunity
There are at least two reasons to be enthusiastic about the sex difference in AVP and AVT systems, or for that matter about any sex difference in the brain. First, the possibility of hormonal manipulation provides a unique perspective for studying how specific neural systems develop. Second, sex differences allow one to study how differences in brain structure translate into differences in function. Solving these questions may also explain why so many behavioral and neurological disorders show marked sex differences (Swaab et al., 2003). This applies to AVP as well because it has been implicated in mood, anxiety, and other behavioral disorders that show marked sex differences (Ring, 2005). However, nature has been reluctant to give up its secrets. Although we know which hormones control sexual differentiation and when they do that, we do not know how. Only a smattering of molecular and cellular processes that mediate hormonal effects on sexual differentiation has been identified (e.g. Amateau and McCarthy, 2004; Auger et al., 2000; Forger et al., 2004), and none of these explain sexual differentiation of AVP and AVT expression. In addition, except for a handful of sexually dimorphic cell groups that control specific sexually dimorphic muscle systems (e.g. the spinal nucleus of the bulbocavernosus, which innervates muscles at the base of the penis; Morris et al., 2004), the functional significance of most sex differences in the CNS is unknown (De Vries and Boyle, 1998). Several features of the AVP/AVT innervation of the brain make it an ideal system to tackle some of these issues.
With AVP being one of the first two peptides to be named a neuropeptide (de Wied, 2000), hundreds of studies have addressed the functions of central AVP and AVT pathways. These functions include, for example, learning and memory (de Wied et al., 1993), reproductive and other social behaviors (Goodson and Bass, 2001; Panzica et al., 2002; Rose and Moore, 2002; Young and Wang, 2004). More recently, studies have exploited the sexually dimorphic and steroid-sensitive character of this system (e.g. Dantzer, 1998; Pittman et al., 1998). Interestingly, this system may cause as well as prevent sex differences in centrally regulated functions and behavior (De Vries and Boyle, 1998), the latter presumably to compensate for sex differences in physiology that, if left unchecked, could cause undesirable differences (De Vries, 2004). Also, with AVP-secreting neurons being relatively easily accessible for electrophysiological, biochemical, and molecular analysis, much is known about cellular and molecular aspects of AVP/AVT expression, release, and neurotransmission (Watters et al., 1998; Young and Gainer, 2003). Given that sexual differentiation of this system depends on phenotypic differentiation, this knowledge should help in identifying where phenotypic decisions may be made. Finally, with many laboratories studying comparative aspects of this system (Goodson and Bass, 2001; Moore and Lowry, 1998; Panzica et al., 2001; Rose and Moore, 2002), the general applicability of knowledge on development and function of its dimorphism may become clearer than it is for most other sex differences in the brain. Comparative studies may also reveal more differences as extreme as those found in Japanese quail and chicken, which should make identifying factors underlying differentiation of these systems easier. This confluence of behavioral, physiological, cellular and molecular, and comparative studies, therefore, creates a unique opportunity to deepen our understanding of the development and functional significance of sex differences in the brain.
Acknowledgments
We thank Nancy Forger for her helpful suggestions. This review was written with support from NIH grants RO1-MH47538 and K02- MH01497 (G.J.D.) and the University of Torino and Regione Piemonte (G.C.P.).
Abbreviations
- AVP
vasopressin
- AVP-ir
vasopressin-immunoreactive
- AVT
vasotocin
- AVT-ir
vasotocin-immunoreactive
- BST
bed nucleus of the stria terminalis
- MeA
medial nucleus of the amygdala
- POM
medial preoptic nucleus
- PVN
paraventricular nucleus of the hypothalamus
- SCN
suprachiasmatic nucleus
REFERENCES
- Absil P, Papello M, Viglietti-Panzica C, Balthazart J, Panzica G. The medial preoptic nucleus receives vasotocinergic inputs in male quail: a tract-tracing and immunocytochemical study. J Chem Neuroanat. 2002;24:27–39. doi: 10.1016/s0891-0618(02)00017-0. [DOI] [PubMed] [Google Scholar]
- al Shamma HA, De Vries GJ. Neurogenesis of the sexually dimorphic vasopressin cells of the bed nucleus of the stria terminalis and amygdala of rats. J Neurobiol. 1996;29:91–98. doi: 10.1002/(SICI)1097-4695(199601)29:1<91::AID-NEU7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Albers HE, Rowland CM, Ferris CF. Arginine-vasopressin immunoreactivity is not altered by photoperiod or gonadal hormones in the Syrian hamster (Mesocricetus auratus) Brain Res. 1991;539:137–142. doi: 10.1016/0006-8993(91)90696-s. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Aste N, Balthazart J, Absil P, Grossmann R, Mulhbauer E, Viglietti-Panzica C, Panzica GC. Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica) J Comp Neurol. 1998;396:141–157. [PubMed] [Google Scholar]
- Aste N, Muhlbauer E, Grossmann R. Distribution of AVT gene expressing neurons in the prosencephalon of Japanese quail and chicken. Cell Tissue Res. 1996;286:365–373. doi: 10.1007/s004410050706. [DOI] [PubMed] [Google Scholar]
- Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc Natl Acad Sci U S A. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger CA, De Vries GJ. Progestin receptor immunoreactivity within steroid-responsive vasopressin-immunoreactive cells in the male and female rat brain. J Neuroendocrinol. 2002;14:561–567. doi: 10.1046/j.1365-2826.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Axelson JF, Shannon W, Van Leeuwen FW. Immunocytochemical localization of estrogen receptors within neurotensin cells in the rostral preoptic area of the rat hypothalamus. Neurosci Lett. 1992;136:5–9. doi: 10.1016/0304-3940(92)90634-j. [DOI] [PubMed] [Google Scholar]
- Azumaya Y, Tsutsui K. Localization of galanin and its binding sites in the quail brain. Brain Res. 1996;727:187–195. doi: 10.1016/0006-8993(96)00379-4. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Dupiereux V, Aste N, Viglietti-Panzica C, Barrese M, Panzica GC. Afferent and efferent connections of the sexually dimorphic medial preoptic nucleus of the male quail revealed by in vitro transport of DiI. Cell Tissue Res. 1994;276:455–475. doi: 10.1007/BF00343944. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. J Neuroendocrinol. 1993;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bennis M, Tramu AM, Reperant J. Vasopressin- and oxytocin-like systems in the chameleon brain. J Hirnforsch. 1995;36:445–450. [PubMed] [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Jetton AE, Villalba C, Devries GJ. Effects of photoperiod and androgen on pituitary function and neuropeptide staining in Siberian hamsters. Am J Physiol. 1996;271:R64–R72. doi: 10.1152/ajpregu.1996.271.1.R64. [DOI] [PubMed] [Google Scholar]
- Boyd SK. Development of vasotocin pathways in the bullfrog brain. Cell Tissue Res. 1994a;276:593–602. doi: 10.1007/BF00343958. [DOI] [PubMed] [Google Scholar]
- Boyd SK. Gonadal steroid modulation of vasotocin concentrations in the bullfrog brain. Neuroendocrinology. 1994b;60:150–156. doi: 10.1159/000126745. [DOI] [PubMed] [Google Scholar]
- Boyd SK, Moore FL. Sexually dimorphic concentrations of arginine vasotocin in sensory regions of the amphibian brain. Brain Res. 1992;588:304–306. doi: 10.1016/0006-8993(92)91590-b. [DOI] [PubMed] [Google Scholar]
- Boyd SK, Tyler CJ, De Vries GJ. Sexual dimorphism in the vasotocin system of the bullfrog (Rana catesbeiana) J Comp Neurol. 1992;325:313–325. doi: 10.1002/cne.903250213. [DOI] [PubMed] [Google Scholar]
- Brot MD, De Vries GJ, Dorsa DM. Local implants of testosterone metabolites regulate vasopressin mRNA in sexually dimorphic nuclei of the rat brain. Peptides. 1993;14:933–940. doi: 10.1016/0196-9781(93)90069-s. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Pevet P, Masson-Pevet M, Pool CW, De Vries GJ, Canguilhem B, Vivien-Roels B. Seasonal variation in vasopressin innervation in the brain of the European hamster (Cricetus cricetus) Brain Res. 1986;371:193–196. doi: 10.1016/0006-8993(86)90829-2. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Swaab DF, Dogterom J, van Leeuwen FW. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Cell Tissue Res. 1978;186:423–433. doi: 10.1007/BF00224932. [DOI] [PubMed] [Google Scholar]
- Caffé AR, Van Leeuwen FW. Vasopressin-immunoreactive cells in the dorsomedial hypothalamic region, medial amygdaloid nucleus and locus coeruleus of the rat. Cell Tissue Res. 1983;233:23–33. doi: 10.1007/BF00222229. [DOI] [PubMed] [Google Scholar]
- Caffé AR, Van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261:237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- Caffé AR, Van Ryen PC, Van der Woude TP, Van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. J Comp Neurol. 1989;287:302–325. doi: 10.1002/cne.902870304. [DOI] [PubMed] [Google Scholar]
- Castel M, Morris JF. The neurophysin-containing innervation of the forebrain of the mouse. Neuroscience. 1988;24:937–966. doi: 10.1016/0306-4522(88)90078-4. [DOI] [PubMed] [Google Scholar]
- Caverson MM, Ciriello J, Calaresu FR, Krukoff TL. Distribution and morphology of vasopressin-, neurophysin II-, and oxytocin-immunoreactive cell bodies in the forebrain of the cat. J Comp Neurol. 1987;259:211–236. doi: 10.1002/cne.902590204. [DOI] [PubMed] [Google Scholar]
- Crenshaw BJ, De Vries GJ, Yahr P. Vasopressin innervation of sexually dimorphic structures of the gerbil forebrain under various hormonal conditions. J Comp Neurol. 1992;322:589–598. doi: 10.1002/cne.903220412. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Vasopressin, gonadal steroids and social recognition. Prog Brain Res. 1998;119:409–414. doi: 10.1016/s0079-6123(08)61584-8. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, al Shamma HA. Sex differences in hormonal responses of vasopressin pathways in the rat brain. J Neurobiol. 1990;21:686–693. doi: 10.1002/neu.480210503. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Best W, Sluiter AA. The influence of androgens on the development of a sex difference in the vasopressinergic innervation of the rat lateral septum. Brain Res. 1983;284:377–380. doi: 10.1016/0165-3806(83)90019-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav Brain Res. 1998;92:205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Sluiter AA. Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res. 1984;298:141–145. doi: 10.1016/0006-8993(84)91157-0. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain-presence of a sex difference in the lateral septum. Brain Res. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Van Leeuwen FW, Caffé AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Duetz W, Buijs RM, van Heerikhuize HJ, Vreeburg JT. Effects of androgens and estrogens on the vasopressin and oxytocin innervation of the adult rat brain. Brain Res. 1986;399:296–302. doi: 10.1016/0006-8993(86)91519-2. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Wang Z, Bullock NA, Numan S. Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the stria terminalis of rats. J Neurosci. 1994;14:1789–1794. doi: 10.1523/JNEUROSCI.14-03-01789.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wied D. Peptide hormones and neuropeptides: birds of a feather. Trends Neurosci. 2000;23:113. doi: 10.1016/s0166-2236(99)01511-8. [DOI] [PubMed] [Google Scholar]
- de Wied D, Diamant M, Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol. 1993;14:251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Theler JM, Ouarour A, Pevet P, Barberis C, Dreifuss JJ. Regional differences in testosterone effects on vasopressin receptors and on vasopressin immunoreactivity in intact and castrated Siberian hamsters. Brain Res. 1994;638:267–276. doi: 10.1016/0006-8993(94)90659-9. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Tribollet E, Dreifuss JJ. Distribution of neurohypophysial peptides in the guinea pig brain. I. An immunocytochemical study of the vasopressin-related glycopeptide. Brain Res. 1989;496:45–65. doi: 10.1016/0006-8993(89)91051-2. [DOI] [PubMed] [Google Scholar]
- Fabris C, Ballarin C, Massa R, Granato A, Fabiani O, Panzica GC, Cozzi B. The vasotocinergic system in the hypothalamus and limbic region of the budgerigar (Melopsittacus undulatus) Eur J Histochem. 2004;48:367–372. [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Miller MA, Dorsa DM, De Vries GJ. Distribution of small vasopressinergic neurons in golden hamsters. J Comp Neurol. 1995;360:589–598. doi: 10.1002/cne.903600404. [DOI] [PubMed] [Google Scholar]
- Fliers E, Guldenaar SE, van de Wal WN, Swaab DF. Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Res. 1986;375:363–367. doi: 10.1016/0006-8993(86)90759-6. [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, De Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci U S A. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Munoz A, Munoz M, Marin O, Smeets WJ. Ontogeny of vasotocinergic and mesotocinergic systems in the brain of the South African clawed frog Xenopus laevis. J Chem Neuroanat. 1995;9:27–40. doi: 10.1016/0891-0618(95)00063-d. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Smeets WJ. Distribution of vasotocin- and mesotocin-like immunoreactivities in the brain of Typhlonectes compressicauda (Amphibia, Gymnophiona): further assessment of primitive and derived traits of amphibian neuropeptidergic systems. Cell Tissue Res. 1997;287:305–314. doi: 10.1007/s004410050755. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Smeets WJ. Distribution of vasotocin- and mesotocin-like immunoreactivities in the brain of the South African clawed frog Xenopus-laevis. J Chem Neuroanat. 1992a;5:465–479. doi: 10.1016/0891-0618(92)90003-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Smeets WJ. Comparative analysis of the vasotocinergic and mesotocinergic cells and fibers in the brain of two amphibians, the anuran Rana ridibunda and the urodele Pleurodeles waltlii. J Comp Neurol. 1992b;315:53–73. doi: 10.1002/cne.903150105. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol. 2004;473:293–314. doi: 10.1002/cne.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober MS, Sunobe T. Serial adult sex change involves rapid and reversible changes in forebrain neurochemistry. Neuroreport. 1996;7:2945–2949. doi: 10.1097/00001756-199611250-00029. [DOI] [PubMed] [Google Scholar]
- Han TM, De Vries GJ. Neurogenesis of galanin cells in the bed nucleus of the stria terminalis and centromedial amygdala in rats: a model for sexual differentiation of neuronal phenotype. J Neurobiol. 1999;38:491–498. [PubMed] [Google Scholar]
- Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol. 2003;54:502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- Hermes ML, Buijs RM, Masson-Pevet M, Pevet P. Seasonal changes in vasopressin in the brain of the garden dormouse (Eliomys quercinus L.) J Comp Neurol. 1990;293:340–346. doi: 10.1002/cne.902930303. [DOI] [PubMed] [Google Scholar]
- Hilscher-Conklin C, Conlon JM, Boyd SK. Identification and localization of neurohypophysial peptides in the brain of a caecilian amphibian, Typhlonectes natans (Amphibia: Gymnophiona) J Comp Neurol. 1998;394:139–151. [PubMed] [Google Scholar]
- Hollis DM, Chu J, Walthers EA, Heppner BL, Searcy BT, Moore FL. Neuroanatomical distribution of vasotocin and mesotocin in two urodele amphibians (Plethodon shermani and Taricha granulosa) based on in situ hybridization histochemistry. Brain Res. 2005;1035:1–12. doi: 10.1016/j.brainres.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Hoorneman EM, Buijs RM. Vasopressin fiber pathways in the rat brain following suprachiasmatic nucleus lesioning. Brain Res. 1982;243:235–241. doi: 10.1016/0006-8993(82)90246-3. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Jacobson CD. Ontogeny of arginine vasopressin-like immunoreactivity in the Brazilian opossum brain. Dev Brain Res. 1995;89:11–32. doi: 10.1016/0165-3806(95)00097-w. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: size changes in relation to age and sex. J Clin Endocrinol Metab. 1999;84:4637–4644. doi: 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- Johnston JB. Further contributions to the study of the evolution of the forebrain. J Comp Neurol. 1923;35:337–481. [Google Scholar]
- Jokura Y, Urano A. Extrahypothalamic projection of immunoreactive vasotocin fibers in the brain of the toad, Bufo japonicus. Zool Sci. 1987;4:675–681. [Google Scholar]
- Jurkevich A, Barth SW, Grossmann R. Sexual dimorphism of arg-vasotocin gene expressing neurons in the telencephalon and dorsal diencephalon of the domestic fowl. An immunocytochemical and in situ hybridization study. Cell Tissue Res. 1997;287:69–77. doi: 10.1007/s004410050732. [DOI] [PubMed] [Google Scholar]
- Jurkevich A, Barth SW, Kuenzel WJ, Kohler A, Grossmann R. Development of sexually dimorphic vasotocinergic system in the bed nucleus of stria terminalis in chickens. J Comp Neurol. 1999;408:46–60. doi: 10.1002/(sici)1096-9861(19990524)408:1<46::aid-cne4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kimura T, Okanoya K, Wada M. Effect of testosterone on the distribution of vasotocin immunoreactivity in the brain of the zebra finch, Taeniopygia guttata castanotis. Life Sci. 1999;65:1663–1670. doi: 10.1016/s0024-3205(99)00415-4. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Voorhuis TA, van Eekelen JA, de Kloet ER, de WD. Organization of vasotocin-immunoreactive cells and fibers in the canary brain. J Comp Neurol. 1987;263:347–364. doi: 10.1002/cne.902630304. [DOI] [PubMed] [Google Scholar]
- Lakhdar-Ghazal N, Dubois-Dauphin M, Hermes ML, Buijs RM, Bengelloun WA, Pevet P. Vasopressin in the brain of a desert hibernator, the jerboa (Jaculus orientalis): presence of sexual dimorphism and seasonal variation. J Comp Neurol. 1995;358:499–517. doi: 10.1002/cne.903580404. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behaviour of adult virgin prairie voles: independence from gonadal hormones and vasopressin. J Neuroendocrinol. 1999;11:441–449. doi: 10.1046/j.1365-2826.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Rood BD, De Vries GJ. Unexpected effects of perinatal gonadal hormone manipulations on sexual differentiation of the extrahypothalamic arginine-vasopressin system in prairie voles. Endocrinology. 2005;146:1559–1567. doi: 10.1210/en.2004-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Richardson CF, Zoeller TR, Miller LJ, Muske LE, Moore FL. Neuroanatomical distribution of vasotocin in a urodele amphibian (Taricha granulosa) revealed by immunohistochemical and in situ hybridization techniques. J Comp Neurol. 1997;385:43–70. doi: 10.1002/(sici)1096-9861(19970818)385:1<43::aid-cne3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Maney DL, Erwin KL, Goode CT. Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows. Horm Behav. 2005;48:196–206. doi: 10.1016/j.yhbeh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Marler CA, Boyd SK, Wilczynski W. Forebrain arginine vasotocin correlates of alternative mating strategies in cricket frogs. Horm Behav. 1999;36:53–61. doi: 10.1006/hbeh.1999.1524. [DOI] [PubMed] [Google Scholar]
- Mathieson WB. Development of arginine vasotocin innervation in two species of anuran amphibian: Rana catesbeiana and Rana sylvatica. Histochem Cell Biol. 1996;105:305–318. doi: 10.1007/BF01463933. [DOI] [PubMed] [Google Scholar]
- Mayes CR, Watts AG, McQueen JK, Fink G, Charlton HM. Gonadal steroids influence neurophysin II distribution in the forebrain of normal and mutant mice. Neuroscience. 1988;25:1013–1022. doi: 10.1016/0306-4522(88)90054-1. [DOI] [PubMed] [Google Scholar]
- Miller MA, Devries GJ, al-Shamma HA, Dorsa DM. Decline of vasopressin immunoreactivity and mRNA levels in the bed nucleus of the stria terminalis following castration. J Neurosci. 1992;12:2881–2887. doi: 10.1523/JNEUROSCI.12-08-02881.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Ferris CF, Kolb PE. Absence of vasopressin expression by galanin neurons in the golden hamster: implications for species differences in extrahypothalamic vasopressin pathways. Brain Res Mol Brain Res. 1999;67:28–35. doi: 10.1016/s0169-328x(99)00029-7. [DOI] [PubMed] [Google Scholar]
- Miller MA, Urban JH, Dorsa DM. Steroid dependency of vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Endocrinology. 1989a;125:2335–2340. doi: 10.1210/endo-125-5-2335. [DOI] [PubMed] [Google Scholar]
- Miller MA, Vician L, Clifton DK, Dorsa DM. Sex differences in vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Peptides. 1989b;10:615–619. doi: 10.1016/0196-9781(89)90152-6. [DOI] [PubMed] [Google Scholar]
- Miller MA, Zoeller RT, Dorsa DM. Detection of vasopressin messenger RNA in cells within the bed nucleus of the stria terminalis by in situ hybridization histochemistry. Neurosci Lett. 1988;94:264–268. doi: 10.1016/0304-3940(88)90028-6. [DOI] [PubMed] [Google Scholar]
- Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119:251–260. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Moore FL, Richardson C, Lowry CA. Sexual dimorphism in numbers of vasotocin-immunoreactive neurons in brain areas associated with reproductive behaviors in the roughskin newt. Gen Comp Endocrinol. 2000;117:281–298. doi: 10.1006/gcen.1999.7424. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Balthazart J, Pessatti M, Viglietti-Panzica C. The parvocellular vasotocin system of Japanese quail: a developmental and adult model for the study of influences of gonadal hormones on sexually differentiated and behaviorally relevant neural circuits. Environ Health Perspect. 2002;110(Suppl 3):423–428. doi: 10.1289/ehp.02110s3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzica GC, Garcia-Ojeda E, Viglietti-Panzica C, Thompson NE, Ottinger MA. Testosterone effects on vasotocinergic innervation of sexually dimorphic medial preoptic nucleus and lateral septum during aging in male quail. Brain Res. 1996;712:190–198. doi: 10.1016/0006-8993(95)01386-5. [DOI] [PubMed] [Google Scholar]
- Panzica G, Pessatti M, Viglietti-Panzica C, Grossmann R, Balthazart J. Effects of testosterone on sexually dimorphic parvocellular neurons expressing vasotocin mRNA in the male quail brain. Brain Res. 1999a;850:55–62. doi: 10.1016/s0006-8993(99)02098-3. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Plumari L, Garcia-Ojeda E, Deviche P. Central vasotocin-immunoreactive system in a male passerine bird (Junco hyemalis) J Comp Neurol. 1999b;409:105–117. [PubMed] [Google Scholar]
- Panzica GC, Aste N, Castagna C, Viglietti-Panzica C, Balthazart J. Steroid-induced plasticity in the sexually dimorphic vasotocinergic innervation of the avian brain: behavioral implications. Brain Res Brain Res Rev. 2001;37:178–200. doi: 10.1016/s0165-0173(01)00118-7. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Castagna C, Viglietti-Panzica C, Russo C, Tlemcani O, Balthazart J. Organizational effects of estrogens on brain vasotocin and sexual behavior in quail. J Neurobiol. 1998;37:684–699. doi: 10.1002/(sici)1097-4695(199812)37:4<684::aid-neu15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Pittman QJ, Chen X, Mouihate A, Martin S. Vasopressin-induced antipyresis. Sex- and experience-dependent febrile responses. Ann N Y Acad Sci. 1998;856:53–61. doi: 10.1111/j.1749-6632.1998.tb08312.x. [DOI] [PubMed] [Google Scholar]
- Planas B, Kolb PE, Raskind MA, Miller MA. Sex difference in coexpression by galanin neurons accounts for sexual dimorphism of vasopressin in the bed nucleus of the stria terminalis. Endocrinology. 1995;136:727–733. doi: 10.1210/endo.136.2.7530652. [DOI] [PubMed] [Google Scholar]
- Plumari L, Plateroti S, Deviche P, Panzica GC. Region-specific testosterone modulation of the vasotocin-immunoreactive system in male dark-eyed junco, Junco hyemalis. Brain Res. 2004;999:1–8. doi: 10.1016/j.brainres.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Plumari L, Viglietti-Panzica C, Allieri F, Honda S, Harada N, Absil P, Balthazart J, Panzica GC. Changes in the arginine-vasopressin immunoreactive systems in male mice lacking a functional aromatase gene. J Neuroendocrinol. 2002;14:971–978. doi: 10.1046/j.1365-2826.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- Prasada Rao PD, Kanwal JS. Oxytocin and vasopressin immunoreactivity within the forebrain and limbic-related areas in the mustached bat, Pteronotus parnellii. Brain Behav Evol. 2004;63:151–168. doi: 10.1159/000076241. [DOI] [PubMed] [Google Scholar]
- Propper CR, Jones RE, Lopez KH. Distribution of arginine vasotocin in the brain of the lizard Anolis carolinensis. Cell Tissue Res. 1992;267:391–398. doi: 10.1007/BF00302978. [DOI] [PubMed] [Google Scholar]
- Ring RH. The central vasopressinergic system: examining the opportunities for psychiatric drug development. Curr Pharm Des. 2005;11:205–225. doi: 10.2174/1381612053382241. [DOI] [PubMed] [Google Scholar]
- Rose JD, Moore FL. Behavioral neuroendocrinology of vasotocin and vasopressin and the sensorimotor processing hypothesis. Front Neuroendocrinol. 2002;23:317–341. doi: 10.1016/s0091-3022(02)00004-3. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- Semsar K, Godwin J. Social influences on the arginine vasotocin system are independent of gonads in a sex-changing fish. J Neurosci. 2003;23:4386–4393. doi: 10.1523/JNEUROSCI.23-10-04386.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira PF, Breno MC, Martin del Rio MP, Mancera JM. The distribution of vasotocin and mesotocin immunoreactivity in the brain of the snake, Bothrops jararaca. J Chem Neuroanat. 2002;24:15–26. doi: 10.1016/s0891-0618(02)00016-9. [DOI] [PubMed] [Google Scholar]
- Smeets WJ, Sevensma JJ, Jonker AJ. Comparative analysis of vasotocin-like immunoreactivity in the brain of the turtle Pseudemys scripta elegans and the snake Python regius. Brain Behav Evol. 1990;35:65–84. doi: 10.1159/000115857. [DOI] [PubMed] [Google Scholar]
- Stoll CJ, Voorn P. The distribution of hypothalamic and extrahypothalamic vasotocinergic cells and fibers in the brain of a lizard, Gekko gecko: presence of a sex difference. J Comp Neurol. 1985;239:193–204. doi: 10.1002/cne.902390206. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Chung WC, Kruijver FP, Hofman MA, Hestiantoro A. Sex differences in the hypothalamus in the different stages of human life. Neurobiol Aging. 2003;24(Suppl 1):S1–S16. doi: 10.1016/s0197-4580(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Szot P, Dorsa DM. Differential timing and sexual dimorphism in the expression of the vasopressin gene in the developing rat brain. Brain Res Dev Brain Res. 1993;73:177–183. doi: 10.1016/0165-3806(93)90136-x. [DOI] [PubMed] [Google Scholar]
- Thepen T, Voorn P, Stoll CJ, Sluiter AA, Pool CW, Lohman AH. Mesotocin and vasotocin in the brain of the lizard Gekko gecko.An immunocytochemical study. Cell Tissue Res. 1987;250:649–656. doi: 10.1007/BF00218959. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen F, Caffé R. Vasopressin-immunoreactive cell bodies in the bed nucleus of the stria terminalis of the rat. Cell Tissue Res. 1983;228:525–534. doi: 10.1007/BF00211473. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen FW, Caffé AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325:391–394. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- Viglietti-Panzica C, Anselmetti GC, Balthazart J, Aste N, Panzica GC. Vasotocinergic innervation of the septal region in the Japanese quail: sexual differences and the influence of testosterone. Cell Tissue Res. 1992;267:261–265. [Google Scholar]
- Viglietti-Panzica C, Aste N, Balthazart J, Panzica GC. Vasotocinergic innervation of sexually dimorphic medial preoptic nucleus of the male Japanese quail: influence of testosterone. Brain Res. 1994;657:171–184. doi: 10.1016/0006-8993(94)90965-2. [DOI] [PubMed] [Google Scholar]
- Viglietti-Panzica C, Balthazart J, Plumari L, Fratesi S, Absil P, Panzica GC. Estradiol mediates effects of testosterone on vasotocin immunoreactivity in the adult quail brain. Horm Behav. 2001;40:445–461. doi: 10.1006/hbeh.2001.1710. [DOI] [PubMed] [Google Scholar]
- Viglietti-Panzica C, Montoncello B, Mura E, Pessatti M, Panzica G. Organizational effects of diethylstilbestrol on brain vasotocin and sexual behavior in male quail. Brain Res Bull. 2005;65:225–233. doi: 10.1016/j.brainresbull.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Voorhuis TA, de Kloet ER. Immunoreactive vasotocin in the zebra finch brain (Taeniopygia guttata) Brain Res Dev Brain Res. 1992;69:1–10. doi: 10.1016/0165-3806(92)90116-e. [DOI] [PubMed] [Google Scholar]
- Voorhuis TA, de Kloet ER, de Wied D. Ontogenetic and seasonal changes in immunoreactive vasotocin in the canary brain. Brain Res Dev Brain Res. 1991;61:23–31. doi: 10.1016/0165-3806(91)90110-5. [DOI] [PubMed] [Google Scholar]
- Voorhuis TA, Kiss JZ, de Kloet ER, de Wied D. Testosterone-sensitive vasotocin-immunoreactive cells and fibers in the canary brain. Brain Res. 1988;442:139–146. doi: 10.1016/0006-8993(88)91441-2. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bullock NA, De Vries GJ. Sexual differentiation of vasopressin projections of the bed nucleus of the stria terminals and medial amygdaloid nucleus in rats. Endocrinology. 1993;132:2299–2306. doi: 10.1210/endo.132.6.8504734. [DOI] [PubMed] [Google Scholar]
- Wang Z, De Vries GJ. Testosterone effects on paternal behavior and vasopressin immunoreactive projections in prairie voles (Microtus ochrogaster) Brain Res. 1993;631:156–160. doi: 10.1016/0006-8993(93)91203-5. [DOI] [PubMed] [Google Scholar]
- Wang Z, De Vries GJ. Androgen and estrogen effects on vasopressin messenger RNA expression in the medial amygdaloid nucleus in male and female rats. J Neuroendocrinol. 1995;7:827–831. doi: 10.1111/j.1365-2826.1995.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Moody K, Newman JD, Insel TR. Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus) Synapse. 1997;27:14–25. doi: 10.1002/(SICI)1098-2396(199709)27:1<14::AID-SYN2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Poulin P, Dorsa DM. Steroid hormone regulation of vasopressinergic neurotransmission in the central nervous system. Prog Brain Res. 1998;119:247–261. doi: 10.1016/s0079-6123(08)61573-3. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young WS, III, Gainer H. Transgenesis and the study of expression, cellular targeting and function of oxytocin, vasopressin and their receptors. Neuroendocrinology. 2003;78:185–203. doi: 10.1159/000073702. [DOI] [PubMed] [Google Scholar]
- Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin-immunoreactive and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology. 1994;134:2622–2627. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]