Abstract
Context
There are potential benefits of mind-body techniques on cognitive function because the techniques involve an active attentional or mindfulness component, but this has not been fully explored.
Objective
To determine the effect of yoga on cognitive function, fatigue, mood, and quality of life in seniors.
Design
Randomized, controlled trial comparing yoga, exercise, and wait-list control groups.
Participants
One hundred thirty-five generally healthy men and women aged 65–85 years.
Intervention
Participants were randomized to 6 months of Hatha yoga class, walking exercise class, or wait-list control. Subjects assigned to classes also were asked to practice at home.
Main Outcome Measures
Outcome assessments performed at baseline and after the 6-month period included a battery of cognitive measures focused on attention and alertness, the primary outcome measures being performance on the Stroop Test and a quantitative electroencephalogram (EEC) measure of alertness; SF-36 health-related quality of life; Profile of Mood States; Multi-Dimensional Fatigue Inventory; and physical measures related to the interventions.
Results
One hundred thirty-five subjects were recruited and randomized. Seventeen subjects did not finish the 6-month intervention. There were no effects from either of the active interventions on any of the cognitive and alertness outcome measures. The yoga intervention produced improvements in physical measures (eg, timed 1-legged standing, forward flexibility) as well as a number of quality-of-life measures related to sense of well-being and energy and fatigue compared to controls.
Conclusions
There were no relative improvements of cognitive function among healthy seniors in the yoga or exercise group compared to the wait-list control group. Those in the yoga group showed significant improvement in quality-of-life and physical measures compared to exercise and wait-list control groups.
Mind-body medicine encompasses a range of methodologies, such as yoga, tai-chi, and meditation, that may be beneficial to the health of their practitioners. Yoga is a commonly practiced, mind-body approach that has components centering around meditation, breathing, and activity or postures. In recent US surveys of adults, 7.5% reported having used yoga at least once in their lifetime and 3.8%–5.1% reported having used it in the previous 12 months.1,2 Iyengar yoga, one of the active, or Hatha, yoga techniques, is a system for developing physical and mental well-being through stretching of all muscle groups for strength, flexibility, and physical balance. A person assumes a series of stationary positions that use isometric contraction and relaxation of different muscle groups to create specific body alignments. There is also a deep relaxation component. Iyengar yoga is amenable to easy adaptation for elders through modifications of the poses and the use of props, such as blankets and chairs.
There are at least 2 mechanisms by which the practice of yoga or exercise may improve cognitive ability. Both may serve to improve mood and reduce stress. Lowered mood is associated with declines in cognitive function and hatha yoga has been reported to produce improvements in mood comparable to aerobic exercise,3,4 so this is one potential mechanism. Additionally, the practice of yoga emphasizes body awareness and involves focusing one’s attention on breathing or specific muscles or parts of body, so it is possible that yoga may improve more general attentional abilities. It is not a far leap from Yoga Sutra (1.2), which says that “Yoga is the control of the whirls of the mind (citta),”5 to consider attentional focus as a major aspect of yoga practice. It is unknown whether the attentional practice in yoga would generalize to conventionally assessed attentional function.
Attention is a multifaceted neural process that allows for differential central nervous system processing of information arising from the external or internal environment. Attention is important for the brain to use its limited resources for higher order processing of only certain salient stimuli and not of stimuli or information that may not be relevant. What attention actually consists of continues to be debated since the psychologist William James wrote more than 100 years ago, “Everyone knows what attention is. It is the taking possession by the mind, in clear and vivid form, of one out of what seem several simultaneously possible objects or trains of thought.”6 There are many aspects of and theories about attention.7–9 The attentional and alertness systems are critical components necessary for all aspects of cognition, including memory and language. Attention may be more amenable to change than other aspects of cognition with either pharmacologic treatments (eg, cholinesterase inhibitors in Alzheimer’s disease; behavioral interventions, such as attentional training in the elderly).10–12 The outcome measures for this study were chosen as measures affected by age: alertness, the ability to sustain attention, the ability to focus attention, the ability to divide attention, and the ability to shift attention.10,13–18 We chose to use an attentional battery that has neuroanatomic correlates wherever possible and that did not rely excessively on non-attentional components, although any cognitive test cannot completely isolate a single aspect of cognitive function. Yoga may have an effect on attentional control which is mediated by frontal lobe structures, so we chose one of the primary outcomes that has known relationships to frontal lobe based on lesion studies or functional imaging.19 Because yoga emphasizes alertness, we chose the other primary outcome measure as a physiologic marker of alertness. Besides the other attentional measures, we included measures of reaction time and declarative memory as secondary measures because there are common changes in these measures with aging, although there was not a high likelihood that improvements would be seen in these latter measures with yoga. Mood and quality of life were also included as secondary outcomes because yoga may have an impact on them.
Despite yoga’s wide popularity, there are limited numbers of randomized, controlled yoga studies using objective quantitative outcome measures, and these studies often have small numbers of subjects.20–24 To evaluate the effect of yoga on cognitive function and quality of life in seniors, we performed a randomized, 26-week trial of yoga in healthy seniors and compared it to exercise and wait-list control groups. The wait-list control is to determine if taking a yoga class has any benefit over doing nothing. Inclusion of the aerobic exercise control group is to assess whether physical activity by itself along with class participation may be beneficial. The outcome measures consisted of assessments of cognitive function focusing on attention, alertness, fatigue, mood, stress, quality of life, and physical abilities.
METHODS
Study Design
This was a 6-month, parallel-group, randomized, controlled trial performed in relatively healthy adults aged 65–85 years that had the approval of the Oregon Health and Science University (OHSU) Institutional Review Board. The research was carried out with the ethical standards set forth in the Helsinki Declaration of 1975. All subjects provided written informed consent. After completion of the baseline evaluations, subjects were randomized to 1 of the 3 experimental groups lasting 6 months: yoga class, exercise class, or wait-list control group. To ensure acceptance of the protocol, wait-list subjects were told they could enroll in either a yoga or exercise class after the 6-month period at no cost.
Subjects were randomly assigned to treatment groups in this study with a planned modified minimization scheme to maintain balance across multiple stratification variables with relatively small numbers of subjects. Cohorts were recruited, and then treatment group assignments were made for the entire cohort at one time, which allowed active intervention subjects to begin their exercise and yoga classes at the same time. The stratification variables were age group and gender. Treatment assignment was conducted by the project statistician, who was otherwise uninvolved with the assessments. Initially, all subjects in each of the cohorts were randomly ordered, ensuring this assignment scheme is in fact random. Then, the minimization approach of Taves was used as a starting point.25 This process minimizes the absolute differences between the groups for each possible assignment of the next patient to a treatment group. Where these absolute differences are equal in 2 or 3 groups, the subject was assigned randomly (with probability of being assigned to 1 group equal across groups). The minimization approach was modified to account for randomization within cohorts before treatment assignment.
Subjects
Subjects were recruited through notices in the local newspaper, community sites, and the OHSU newsletter website. Recruitment began in October 1999, and the last cohort of subjects had outcome assessments in June 2003. Prospective participants were screened for significant medical problems with history, physical examination, and routine electrocardiogram to ensure the safety of the intervention and to exclude subjects with an underlying medical illness that might impair cognition. Subjects were excluded for any of the following reasons: insulin-dependent diabetes; uncontrolled hypertension; evidence of liver or kidney failure; significant lung disease; alcoholism or other drug abuse; symptoms or signs of congestive heart failure, symptomatic ischemic heart disease, or significant valvular disease; and significant visual impairment. Subjects also were excluded if they were actively practicing yoga or had taken a yoga or tai-chi class in the last 6 months or if they were regularly performing aerobic exercise more than 210 minutes per week (about half the subjects were engaged in some regular weekly exercise). Subjects spoke English as their primary language. The targeted enrollment was 150 subjects with an estimated power of 0.8 using analysis of covariance (ANCOVA) for the Stroop test, assuming a moderate effect size (f = .25), baseline-6-month visit correlations of 0.5, and a 20% attrition rate.
Interventions
Beginning Iyengar yoga poses were taught to study participants in a gentle way. Based on what is often available in the community and to maintain compliance for a 6-month trial, we set the yoga intervention schedule at 1 class per week along with home practice. The yoga classes were 90 minutes in duration to help ensure a deep enough practice to produce desired effects. The yoga class was designed by a certified Iyengar yoga teacher, an Iyengar trained teacher, and a physician. Eighteen poses were taught, although not all each week. An average of 7–8 poses were taught each week. Repetition was consistent from week to week and linked pose to pose. Each pose was held for approximately 20–30 seconds, with rest periods between poses lasting 30 seconds to 1 minute. A mixture of standing and seated poses were introduced and practiced. Props such as blankets, blocks, and straps were used. Normal breathing for relaxation was emphasized during each session. Participants were encouraged to honor individual limits and hold the pose for less time if necessary. Each class ended with a 10-minute deep relaxation period with the subject lying supine. Progressive relaxation, visualization, and meditation techniques were introduced during this time. Daily home practice was strongly encouraged. Subjects were given a booklet illustrating the specific poses to help with their independent practice.
A nurse who is also a certified personal trainer with experience in the geriatric population directed the aerobic exercise intervention arm of the study. The aerobic intervention, like the yoga intervention, consisted of 1 class per week along with home exercise. The aerobic exercise consisted of walking on an outdoor 400-meter track for endurance training. The 1-hour class began with walking 2 laps to warm up and then progressed to mild leg stretches. Intensity of exercise was determined by heart rate and modified Borg Rate of Perceived Exertion scale, the Borg CR10 Scale.26 Subjects wore a heart-rate monitor, and target heart rate was initially estimated as 70% of maximum based on morning resting heart rate and age. Subjects were instructed to exercise at a level of 6–7 on the Perceived Exertion scale. Based on perceived exertion, the heart rate target was adjusted slightly. Subjects were strongly encouraged to exercise daily at least 5 times per week in addition to the weekly class session.
There was no intervention for the wait-list control subjects. They received the same monthly phone calls to assess for changes in health and underwent the same assessments as the other study participants.
Compliance with the interventions was assessed by having study participants complete daily 2-week log sheets that recorded whether or not they exercised or practiced yoga and for how long. Class attendance also was recorded.
Assessments
After medical history was reviewed and a physical exam and routine electrocardiogram were conducted, baseline assessments of outcome measures were performed. Baseline assessments were performed before subjects were randomized and occurred 1 to 30 days before the classes started. The practical limitations on the rate of testing participants required 5 separate cohorts of subjects. Each cohort contained 20–32 subjects, each of whom was assigned to 1 of the 3 intervention arms. All outcome assessments were done at baseline and 6 months. There was also a 3-month visit primarily designed to encourage subjects’ continued participation in the study, but most outcome measures were not obtained at this mid-study visit. On the baseline visit, demographic data were recorded, and the oral reading on the Wide Range Achievement Test (WRAT), 3rd edition, was administered to assess equality of educational achievement in the 3 intervention groups.27
It was important to plan carefully to maintain blinding of the assessors generating the outcome measures because the subjects were non-blinded. Only a single liaison person who was responsible for direct phone calls to subjects was unblinded, and this person did not participate in one-on-one testing after randomization (ie, after the baseline assessment). The 3- and 6-month assessments were planned carefully to ensure continued blinding. Some of the outcome measures were done independently of any assessor (ie, with self-rating forms). For the in-person evaluations, the liaison person scheduling the appointment instructed the subjects to not tell the assessor what intervention group they were in. A reminder call was made the day before the assessments, and subjects were reminded to not speak about their intervention group. Even with these precautions, there were rare instances of un-blinding. Fortunately, the assessments were objective, and many were computer-based and -scored. The blinded research staff maintained equipoise about potential results of the study. The data analysis was blinded to intervention group.
The baseline and outcome sets of cognitive assessments were performed at the same time of day for each subject—at their preferred time, either in the morning or in the afternoon. The subjects were instructed to refrain from alcohol consumption for 24 hours before the testing. They were allowed to ingest their usual dose of morning caffeine.
Attention and Alertness
The primary outcome measures were The Stroop Color and Word Test28 and a quantitative electroencephalogram (EEC) measure of alertness (posterior median power frequency). Obtaining reliable data on elderly subjects required the testing session to be limited to 2 hours to prevent excessive fatigue. Due to this constraint, some excellent neuropsychological tests of attention were not included. The cognitive assessments focused on aspects of attention (focusing attention, shifting attention, dividing attention, and sustaining attention) that may be altered with aging and were also thought to be most likely to be improved with the intervention. The Stroop Color and Word Test,28 Victoria version, color-word interference in seconds was used as a measure of ability to focus attention. The covert orienting of spatial attention task compares reaction time (RT) when targets are validly cued, neutrally cued, invalidly cued, or not cued.29–31 Median RTs were calculated for the 4 cue conditions, although only the invalid-valid RT difference, a measure of ability to shift spatial attention, was used as an outcome measure of shifting attention ability. Another attentional shifting task used was adapted from that used in the Cambridge Neuropsychological Test Automated Battery and is related to the Wisconsin Card-Sorting Test. It allows attentional shifting to be broken down into 3 types: intra-dimensional, reversal, and extra-dimensional.32 The outcome measure was the number of shifts correctly performed before completion of the test. A modified Useful Field of View task was chosen as a divided attention test because it has some ecologic validity in relationship to driving ability in seniors.33,34 Our modification determined a precise temporal threshold. Subjects with automated threshold determinations above 600 msec were considered test administration failures. Additionally, the percentage of errors made at time durations above threshold was determined as a measure of sustained attention. Simple and choice visual RT was measured as we have previously done.23
In case there were effects of the intervention on alertness and attention, we performed other cognitive tasks to determine the specificity of the effect: a 10-word list learning task35 (delayed memory adjusted for immediate recall); and the Wechsler Adult Intelligence Scale III (WAIS-III) Letter-Number Sequencing36 to assess working memory.
Two subjective scales were used to measure alertness, the Stanford Sleepiness Scale (SSS)37 and the Profile of Mood States (POMS),38 which have shown to be sensitive to drug effects.31,39 We used the POMS as a mood measure as well because it has been reported to show improvement with an exercise intervention in a multiple sclerosis study40 and with a Hatha yoga intervention in young adults.3
Electroencephalogram for frequency analysis as a measure of alertness was acquired as previously described.31,39 The only EEC frequency analysis measure used for the analysis was the posterior median power frequency recorded during an eyes-closed attentive state.
Mood, Fatigue, and Quality of Life
Mood, including measures of fatigue and vigor, was assessed using the POMS.38 Fatigue was also assessed using the Multidimensional Fatigue Inventory (MFI).41 Depression was assessed by the POMS and the Center for Epidemiologic Studies Depression Scale (CESD-10).42 Health-related quality of life was assessed by the SF-36.43 The MFI, POMS, State-Trait Anxiety Inventory (STAI) and SF-36 were filled out by the subjects at home and reviewed by the research assistant at the time of the cognitive testing to help minimize duration of the assessment session.
Physical Measures
Subjects performed a measure of forward-bend flexibility, the chair sit, and reach.44 Subjects were asked to stand as long as they could on each leg with their eyes open,45 and the duration was averaged across the 2 legs. Subjects performed a timed measure to stand and sit 5 times.46
Data Analysis
The primary analysis used all randomized subjects who had follow-up data (ie, those who completed the 6-month study). No attempt was made to impute missing variables for subjects from whom only baseline data was available.
Most of the outcome data were analyzed using an ANCOVA approach with baseline value as the covariate, indicator variables for each of the 2 active groups (ie, yoga and exercise), and the interactions of the indicators with baseline. In addition, 2 baseline factors (age and gender) were evaluated as potential confounding variables. The numeric value of age was included in these models rather than grouping the numeric values into categories. If the data could not be normalized (as with some of the SF-36 subscales), non-parametric measures were used, including grouping responses together and using logistic regression.
The following approach was used to determine the “best” ANCOVA model for each response. Backward variable elimination determined which among baseline, age, and an indicator for gender were significant predictors. To control for potential confounding variables, the following approach was used. Any baseline predictor significant at .10 was included in the next stage of model fitting. Second, indicator variables for the 2 active groups and the interactions of these indicators with baseline predictors already in the model were added to the best model above. Partial F-tests were used to test whether or not the 2 interaction terms were simultaneously equal to zero. If the interactions were not significant, partial F-tests were also used to simultaneously test whether the 2 group indicators were simultaneously equal to zero. If either hypothesis was rejected, backward elimination was used to eliminate any individual terms that were not significant (using a significance level of .05). Residuals from the best model were assessed to determine whether normality was violated (in particular if there was a substantial skew). If so, one or more transformations (the natural logarithm, square root, or the rank transformation, in order) were evaluated by following this same approach.
The primary outcome measures were assessment of alertness based on EEG median power frequency and assessment of attention by color-word interference on the Stroop Color and Word Test. No Bonferroni corrections were made for multiple outcome measures. The secondary measures were the rest of the cognitive assessments, self-rated scales (MFI, POMS, CESD-10, SF-36), and the physical measures.
RESULTS
Following screening of 268 subjects, 135 eligible subjects gave informed consent and were randomized to 1 of 3 groups (Figure 1). Forty-one potentially eligible subjects declined the study for various reasons, including practical issues (eg, couldn’t attend a weekly class, too far a drive to the class site) and not wanting to accept randomization (eg, wanted to start a yoga class). Characteristics of the enrolled subjects are shown in Table 1. Seventeen subjects did not complete the 6-month intervention. The 12.6% dropout rate was not related to adverse events, as there were no significant adverse events related to the intervention. The dropout rate was slightly lower in the wait-list control group but the difference in dropout rates as assessed by Pearson chi-square was not significant (P = .107). The only adverse events possibly related to the intervention were a groin muscle strain in a yoga-group subject and increased hip pain in an exercise-group subject who had a short leg. The most common cause for dropping out of the study was dissatisfaction with the assignment to wait-list or exercise, family health issues, and time constraints. There were several dropouts related to dissatisfaction with the randomization group. These occurred despite the subjects having been given a clear explanation of the randomization process and the subjects having to specifically verbally consent to accept the random assignment to 1 of the 3 groups in addition to signing the consent form, which also contained randomization information.
FIGURE 1.
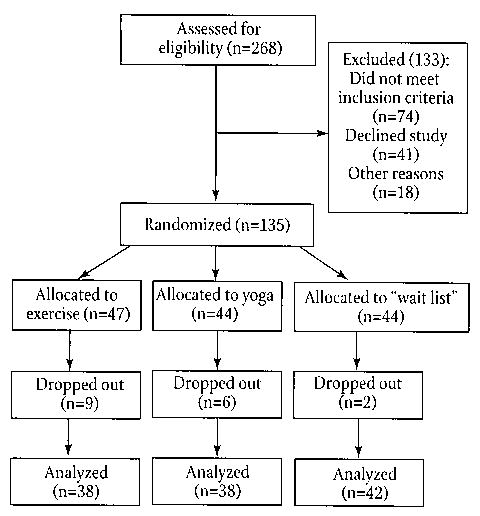
Flow of Study Subjects From Recruitment Through Randomization and Analysis
TABLE 1.
Baseline Characteristics of the Study Population
| Yoga (n=44) | Exercise (n=47) | Wait list (n=44) | |
|---|---|---|---|
| Women | 31 | 37 | 33 |
| Men | 13 | 10 | 11 |
| Race: White | 42 | 36 | 38 |
| Asian-American | 4 | 7 | 5 |
| Black | 1 | 1 | 1 |
| Age | 71.5 ± 4.9 | 73.6 ± 5.1 | 71.2 ± 4.4 |
| Years of education | 15.4 ± 2.2 | 14.8 ± 2.8 | 15.3 ± 2.8 |
| WRAT-3 | 48.7 ± 4.3 | 49.0 ± 4.1 | 49.0 ± 3.4 |
| BMI | 25.7 ± 5.7 | 27.5 ± 4.4 | 26.4 ± 5.3 |
WRAT=Wide Range Achievement Test, 3rd edition, oral reading subtest; BMI=body mass index
Of all subjects completing the 6-month exercise intervention arm, the attendance rate at the weekly classes was 69%, subjects exercised an average of 54% of all days, and the average length of exercise when done was 56 minutes. For the group completing the 6-month yoga intervention, the attendance rate was 78%, and home practice occurred on 64% of the days and averaged 38 minutes (Table 2).
TABLE 2.
Compliance
| Classes attended (%) | Days practiced (%) | Average practice length (min) | |
|---|---|---|---|
| Exercise | 68.7 + 18.7 | 54 + 26 | 56.1 + 26.4 |
| Yoga | 77.6 + 19.7 | 64 + 21 | 37.8 + 10.4 |
Means and standard deviations for 3 compliance measures for the active intervention groups.
There was no effect of assignment group on any of the cognitive function or alertness measures, which included the primary outcome measures for this study (Table 3).
TABLE 3.
Baseline and 6-month Outcomes Data on All Subjects for Whom Both Data Points Were Available
| Yoga | Exercise | Wait list | |||||
|---|---|---|---|---|---|---|---|
| Outcome Measures | Baseline | 6-month | Baseline | 6-month | Baseline | 6-month | Pvalue |
| Attention and alertness | |||||||
| Stroop Interference (sec) primary | 11.1 ± 7.3 | 10.0 ± 4.6 | 11.4 ± 5.3 | 10.8 ± 4.3 | 12.6 ± 6.4 | 11.0 ± 3.7 | .72 |
| EEC Auditory MPF (Hz) primary | 9.9 ± 1.1 | 9.7 ± 1.1 | 9.5 ± 1.1 | 9.5 ± 0.9 | 10.1 ± 0.9 | 10.0 ± 1.0 | .46 |
| Word list delayed recall | 7.3 ± 1.8 | 7.2 ± 1.9 | 6.5 ± 2.0 | 7.0 ± 2.0 | 6.6 ± 1.8 | 7.1 ± 1.6 | .38 |
| Letter-number sequencing | 10.0 ± 2.2 | 10.5 ± 2.6 | 10.2 ± 3.0 | 10.7 ± 2.8 | 10.1 ± 2.7 | 10.8 ± 4.0 | .94 |
| Covert orienting (invalid-valid) | 21.4 ± 29.3 | 40.3 ± 36.8 | 25.4 ± 33.3 | 37.1 ± 34.1 | 25.9 ± 29.4 | 36.6 ± 30.0 | .76 |
| Divided attention threshold (msec) | 102.0 ± 64.4 | 89.8 ± 43.2 | 122.0 ± 69.6 | 111.8 ± 67.6 | 106.2 ± 55.8 | 92.3 ± 47.6 | .48 |
| % errors above threshold | 5.5% ± 3.8% | 4.9% ± 3.6% | 6.0% ± 3.6% | 5.0% ± 2.9% | 7.3% ± 3.3% | 6.2% ± 3.4% | .45 |
| Set shifting: highest shift | 8.9 ± 2.0 | 9.5 ± 1.3 | 9.1 ± 1.8 | 9.9 ± 0.4 | 9.2 ± 1.9 | 9.2 ± 1.8 | .14 |
| Simple reaction time (msec) | 316.6 ± 65.0 | 335.6 ± 74.0 | 302 ± 89.4 | 321.9 ± 79.7 | 296 ± 75.1 | 311.4 ± 78.5 | .76 |
| Choice reaction time (msec) | 494.8 ± 57.1 | 491.9 ± 49.1 | 493.4 ± 74.2 | 487.2 ± 63.9 | 483.3 ± 50.6 | 469.0 ± 48.2 | .52 |
| Stanford sleepiness scale | 1.6 ± 0.8 | 2.0 ± 0.9 | 1.8 ± 1.0 | 2.1 ± 0.9 | 1.6 ± 0.7 | 1.9 ± 0.9 | .75 |
| Mood, quality of life | |||||||
| SF-36 Physical Function | 84.3 ± 13.8 | 84.9 ± 14.6 | 82.6 ± 12.4 | 82.2 ± 15.3 | 88.2 ± 9.6 | 86.7 ± 9.6 | .62 |
| Role—physical*** | 80.3 ± 29.7 | 94.1 ± 15.8 | 77.0 ± 32.6 | 65.1 ± 41.3 | 78.7 ± 31.4 | 74.4 ± 35.1 | .001 |
| Bodily pain** | 75.8 ± 19.6 | 79.5 ± 17.7 | 73.7 ± 19.3 | 68.4 ± 24.3 | 70.5 ± 21.3 | 65.9 ± 20.3 | .006 |
| General health | 81.5 ± 17.0 | 82.8 ± 13.8 | 80.4 ± 13.9 | 80.8 ± 16.1 | 80.0 ± 13.4 | 81.3 ± 15.3 | .54 |
| Social functioning* | 93.8 ± 14.7 | 95.1 ± 12.2 | 90.1 ± 14.6 | 82.9 ± 21.4 | 91.9 ± 13.4 | 90.9 ± 16.5 | .015 |
| Role—emotional | 84.2 ± 28.7 | 92.1 ± 23.8 | 79.8 ± 29.6 | 83.3 ± 32.7 | 81.7 ± 30.1 | 85.8 ± 27.1 | .25 |
| Mental health | 83.7 ± 13.7 | 88.3 ± 9.8 | 84.6 ± 11.1 | 84.1 ± 16.9 | 84.7 ± 11.5 | 87.2 ± 8.8 | .88 |
| Vitality** | 71.1 ± 17.4 | 75.4 ± 17.3 | 67.4 ± 14.5 | 62.2 ± 20.0 | 75.3 ± 12.9 | 74.9 ± 12.4 | .006 |
| Physical composite** | 49.9 ± 6.8 | 51.1 ± 6.3 | 48.9 ± 7.3 | 46.8 ± 9.7 | 50.0 ± 6.1 | 47.9 ± 6.3 | .005 |
| Mental composite | 55.1 ± 6.5 | 57.3 ± 5.6 | 54.3 ± 7.3 | 53.9 ± 9.8 | 55.0 ± 6.9 | 56.7 ± 6.0 | .61 |
| CESD-10 | 5.1 ± 3.8 | 4.0 ± 2.8 | 4.8 ± 3.9 | 6.3 ± 6.0 | 4.6 ± 3.4 | 4.0 ± 3.0 | .91 |
| POMS Depression-dejection | 5.0 ± 6.1 | 3.1 ± 4.1 | 3.7 ± 4.7 | 4.4 ± 7.5 | 4.4 ± 5.2 | 3.4 ± 3.1 | .68 |
| Vigor—activity | 21.0 ± 5.7 | 15.5 ± 10.3 | 19.9 ± 3.8 | 12.5 ± 9.5 | 21.9 ± 6.0 | 14.5 ± 9.7 | .55 |
| Fatigue—inertia | 4.2 ± 4.2 | 1.5 ± 2.0 | 4.3 ± 3.5 | 3.2 ± 4.5 | 3.4 ± 3.8 | 2.6 ± 3.3 | .30 |
| MFI general fatigue | 8.1 ± 3.3 | 7.8 ± 3.6 | 8.8 ± 3.7 | 9.7 ± 3.7 | 7.7 ± 3.1 | 7.9 ± 3.1 | .18 |
| Physical fatigue | 7.8 ± 3.2 | 7.5 ± 3.2 | 7.7 ± 2.9 | 8.6 ± 3.6 | 6.9 ± 2.7 | 7.4 ± 3.0 | .35 |
| Reduced activity* | 8.0 ± 3.5 | 7.0 ± 3.0 | 8.1 ± 3.6 | 8.9 ± 3.3 | 7.2 ± 3.5 | 7.6 ± 3.7 | .015 |
| Reduced motivation | 6.7 ± 2.8 | 6.5 ± 2.7 | 7.0 ± 2.6 | 7.4 ± 3.4 | 6.2 ± 2.5 | 6.5 ± 2.6 | .69 |
| Mental fatigue | 7.7 ± 3.5 | 7.7 ± 3.1 | 7.7 ± 3.5 | 8.6 ± 3.8 | 7.1 ± 3.0 | 7.0 ± 2.5 | .24 |
| Physical measures | |||||||
| One-leg stand (sec)* | 29.6 ± 41.5 | 39.2 ± 52.3 | 11.1 ± 11.9 | 12.8 ± 14.4 | 21 .4 ± 30.1 | 24.2 ± 21.5 | .049 |
| Chair sit and reach (cm)* | 1.2 ± 15.5 | 6.4 ± 12.7 | 0.1 ± 12.3 | −0.1 ± 14.6 | −0.3 ± 13.3 | −3.3 ± 14.7 | .016 |
| Sit and stand (sec) | 10.4 ± 2.9 | 9.2 ± 2.5 | 9.9 ± 2.2 | 10.0 ± 2.6 | 9.4 ± 2.5 | 9.1 ± 2.1 | .50 |
| ¼-mile walk (sec) | 154.7 ± 132.5 | 140.7 ± 127.8 | 179.5 ± 134.2 | 188.1 ± 138.0 | 151.9 ± 127.0 | 143.1 ± 125.5 | .26 |
First two measures (ital) were primary outcome measures. EEG=electroencephalogram; MPF=median power frequency, CESD= Center for Epidemiotogic Studies of Depression, POMS=Profile of Mood States, MFI=Multidimensional Fatigue Inventory
P<.05,
P<.01,
P<.001
The yoga intervention group members rated themselves significantly better than the exercise group or the wait-list control group on several measures. The SF-36 quality-of-life measure demonstrated a significant yoga assignment group effect on vitality/energy and fatigue (P = .006), role-physical (P = .001), bodily pain (P = .006), social functioning (P = .015), and the physical composite scale (P = .005). There were no effects on mood as assessed with POMS or CESD-10. On the MFI, reduced activity was better in the yoga group (P = .015), but there was no difference in the other subscales.
The yoga group also demonstrated improvements in physical measures. Timed one-legged standing was improved (P < .05) as was seated forward bending (P < .05). There were no physical measures implemented at study onset to assess the exercise intervention.
DISCUSSION
This is the largest, most carefully controlled trial of yoga in seniors conducted to date. The trial demonstrated that a 6-month yoga program did not produce any improvements in cognitive function. There were significant improvements in quality-of-life measures. There were also significant improvements in outcome measures specifically related to the intervention (eg, one-legged standing and seated forward-bending ability).
The subjects in the yoga and exercise intervention did not do better than the wait-list control group on the cognitive outcome measures, but this finding must be interpreted cautiously. Though exercise interventions in animals have been reported to show benefits to brain function and structure,47 the effect of a short-term randomized exercise intervention exercise on cognitive function in healthy seniors is not large, with the differences perhaps related to the intensity of the exercise intervention. Although most cross-sectional studies have suggested exercise is beneficial to cognitive function,48–51 data from exercise intervention studies are less clear.52,53 There have been several intervention studies with clear improvement in VO-2 max (ie, maximal rate of oxygen consumption) or other physiologic parameters, mood, and quality of life without a clear improvement in cognitive function.54,55 Additionally, exercise intervention may improve only certain aspects of cognitive function.56–58 Healthy seniors such as those who volunteered for this study may be functioning near their best and may not be able to demonstrate significant improvement during a 6-month study. This may contrast with seniors who are potentially not at their best—beneficial effects from exercise interventions in seniors with depression have been more consistent.59,60
The improvements in physical measures directly related to the yoga intervention are not surprising. Yoga practice involves training on poses very similar to these outcome measures. One-legged balance may have some health implications, such as risk of falls, and has been shown previously to be improved in healthy older people practicing tai chi, another mind-body technique of which balance exercises are a component.61,62
Though this study did show that yoga produced beneficial effects on quality-of-life measures, the mechanism of action of these improvements may not relate directly to the yoga. Socialization, placebo, and self-efficacy effects are other potential mechanisms. The exercise group controlled for socialization to some degree, but there was less of a class format in the exercise group. At least 1 previous study has suggested that exercise-related improvements in stress were secondary to class participation and not to improvements in fitness.63 Future yoga intervention studies will need to carefully control for the class aspect that may be beneficial to everyone, but especially seniors. There is also likely some placebo effect related to the yoga intervention. One group has already shown that psychological benefits of an aerobic exercise intervention in a group of healthy young adults could be increased simply by telling subjects that the exercise program was specifically designed to improve psychological well-being.64 The placebo effect, expectancy, and self-efficacy may have a significant impact65,66 and are difficult to adequately control for in behavioral interventions that are necessarily non-blinded. Even reported cognitive improvements related to transcendental meditation may be related to expectancy of subjects recruited for trials.67
Although there were many secondary outcome measures, we do not believe the findings are simply random results from multiple comparisons. The P value for the intervention effect on one of the SF-36 subscales was sufficiently low that it would have been significant even with a very conservative Bonferroni correction. However, these were secondary outcome measures, so these findings should be considered preliminary. Also, given power issues and size, the absence of statistically significant effects on the mood measures needs to be interpreted conservatively, and there remains a possibility that mood improvements contributed to these improvements in quality of life and fatigue. For example, given the correlation coefficient for baseline and 6-month CESD-10 of 0.4, the study was powered at only 0.8 to detect an effect of about 1 point.
Regarding the lack of effect on quality-of-life measures in the exercise group, most other randomized exercise intervention studies included only seniors who were consistently more sedentary than those in this study. Because the exercise class was primarily serving as a control group for the yoga intervention, we allowed subjects at entry to be doing aerobic exercise up to 3.5 hours per week (30 minutes per day). The seniors were more physically active at baseline than were subjects with multiple sclerosis in a concurrently performed 3-armed yoga and exercise intervention study in which the exercise intervention produced improvements in fatigue and quality of life comparable to those produced by yoga.23 In that study, there were also no effects on the cognitive outcome measures from either intervention. The exercise class in the current study was just once per week, in contrast to more frequent classes in most other exercise intervention studies in seniors.60,68 Additionally, in this study, the yoga intervention was considered more desirable by many of our participants, so there may be effects related to disappointment and expectancy, as well.
There are many issues related to design of a yoga or exercise intervention that are significantly different from the usual drug study and may affect generalizability. For future trial planning, these are mentioned briefly. Class scheduling logistics needed to address the issue of preferred times for each cohort of subjects. The yoga class was adapted for beginning seniors; thus, the results of this study may not be directly generalizable to a typical community yoga class. The other potential issue related to generalizability is that our subjects were a highly motivated group that was willing to volunteer for a research study. Subjects are necessarily non-blinded for these interventions, and there was a significant discussion at the time informed consent was obtained about the randomization process and the need for subjects to accept the randomization. Even with this measure, several subjects dropped out, probably because of disappointment with their randomized group assignment.
Healthy seniors participating in a 6-month yoga or exercise class showed no differences in cognitive function compared to a wait-list control group. The yoga intervention produced improvements in quality-of-life measures not seen in the exercise group and also improvements in physical measures related to the intervention itself (eg, timed 1-legged standing and forward-bending flexibility). The lack of effect on cognitive function does not necessarily imply that yoga or aerobic exercise is not beneficial to cognition but may relate to ceiling effects in this relatively healthy group of seniors or the relatively short intervention period of the study.
Acknowledgments
This study was supported by National Institutes of Health grants AT-00066 and Grant 5 M01 RR000334. Portions of this paper were presented at the 2004 American Academy of Neurology meeting. We would like to acknowledge Roger Ellingson, who provided computer programming support, and Drs Kaleeswari Arulselvam and Magda Rittenbaum, who performed medical evaluations and assisted in participant testing.
References
- 1.Saper RB, Eisenberg DM, Davis RB, Culpepper L, Phillips RS. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10:44–49. [PubMed] [Google Scholar]
- 2.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. CDC Ad Data. 2004;(343) May 27, 2004:1–19. [PubMed]
- 3.Berger BG, Owen DR. Mood alteration with yoga and swimming: aerobic exercise may not be necessary. Percept Mot Skills. 1992;75(3 Pt 2):1331–1343. doi: 10.2466/pms.1992.75.3f.1331. [DOI] [PubMed] [Google Scholar]
- 4.Berger BG, Owen DR. Stress reduction and mood enhancement in four exercise modes: swimming, body conditioning, Hatha yoga, and fencing. Res Q Exerc Sport. 1988;59(2):148–159. [Google Scholar]
- 5.Feuerstein G. Traditional definitions of yoga. Yoga Research and Education Center website. Available at: http://www.yrec.info/contentid-l7.html Accessed November 29, 2005.
- 6.James W. The Principles of Psychology. Vol 1. New York: Henry Holt; 1890.
- 7.Parasuraman R, ed. The Attentive Brain. Cambridge, MA: The MIT Press; 1998.
- 8.Pashler HE. The Psychology of Attention. Cambridge: MIT Press; 1997.
- 9.Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 10.Hertzog CK, Williams MV, Walsh DA. The effect of practice on age differences in central perceptual processing. J Gerontol. 1976;31:428–433. doi: 10.1093/geronj/31.4.428. [DOI] [PubMed] [Google Scholar]
- 11.Parasuraman R, Giambra L. Skill development in vigilance: effects of event rate and age. Psychol Aging. 1991;6:155–169. doi: 10.1037//0882-7974.6.2.155. [DOI] [PubMed] [Google Scholar]
- 12.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carskadon MA. Ontogeny of human sleepiness as measured by sleep latency In: Dinges DF, Broughton RJ, eds. Sleep and Alertness: Chronobiological, Behavioral, and Medical Aspects of Napping. New York: Raven Press; 1989:53–69.
- 14.Oken BS, Salinsky M. Alertness and attention: basic science and electrophysiologic correlates. J Clin Neurophysiol. 1992;9:480–494. [PubMed] [Google Scholar]
- 15.Rabbitt P. An age-decrement in the ability to ignore irrelevant information. J Gerontol. 1965;20:233–238. doi: 10.1093/geronj/20.2.233. [DOI] [PubMed] [Google Scholar]
- 16.Hanninen T, Hallikainen M, Koivisto K, et al. Decline of frontal lobe functions in subjects with age associated memory impairments. Neurology. 1997;48:148–153. doi: 10.1212/wnl.48.1.148. [DOI] [PubMed] [Google Scholar]
- 17.Ball K, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. J Am Optom Assoc. 1992;63:71–79. [PubMed] [Google Scholar]
- 18.Sahakian BJ, Downes JJ, Eagger S, et al. Sparing of attentional relative to mnemonic function in a subgroup of patients with dementia of the Alzheimer type. Neuropsychologia. 1990;28(11):1197–1213. doi: 10.1016/0028-3932(90)90055-s. [DOI] [PubMed] [Google Scholar]
- 19.Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: An H2 15O PET study of stroop task performance. Neuroimage. 1995;2:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- 20.Garfinkel MS, Singhal A, Katz WA, Allan DA, Reshetar R, Schumacher HR., Jr Yoga-based intervention for carpal tunnel syndrome. JAMA. 1998 November 11;280(18):1601–1603. doi: 10.1001/jama.280.18.1601. [DOI] [PubMed] [Google Scholar]
- 21.Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial ot the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253–2260. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 22.Ramaratnam S, Sridharan K. Yoga for epilepsy. Cochrane Database of Syst Rev. 2005(4).
- 23.Oken BS, Kishiyama S, Zajdel D, et al. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology. 2004;62:2058–2064. doi: 10.1212/01.wnl.0000129534.88602.5c. [DOI] [PubMed] [Google Scholar]
- 24.Riley D. Hatha yoga and meditation. In: Oken BS, ed. Complementary Therapies in Neurology: an evidence-based approach. London, UK: Parthenon Publishing; 2004:159–167.
- 25.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15:443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 26.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
- 27.Wilkinson GS. Wide Range Achievement Test 3. Wilmington, Del: Wide Range, Inc; 1993.
- 28.Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- 29.Posner MI. Orienting of attention. QJ Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 30.Oken BS, Kishiyama SS, Kaye JA, Howieson DB. Attention deficit in Alzheimer’s disease is not simulated by an anticholinergic/anithistaminergic drug and is distinct from deficits in healthy aging. Neurology. 1994;44:657–662. doi: 10.1212/wnl.44.4.657. [DOI] [PubMed] [Google Scholar]
- 31.Griesar WS, Zajdel DP, Oken BS. Nicotine effects on alertness and spatial attention in non-smokers. Nicotine Tob Res. 2002;4:185–194. doi: 10.1080/14622200210123617. [DOI] [PubMed] [Google Scholar]
- 32.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 33.Ball KK, Roenker DL. UFOV: Useful Field of View, San Antonio, Tex: The Psychological Corporation, Harcourt Brace & Company; 1998.
- 34.Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Investigative Ophthalmology & Visual Science. 1993;34:3110–3123. [PubMed] [Google Scholar]
- 35.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part 1. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D. Wechsler Adult Intelligence Scale. Third ed. San Antonio, Tex: The Psychological Corporation; 1997.
- 37.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 38.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego: EdITS/Educational and Industrial Testing Service; 1992.
- 39.Oken BS, Kishiyama SS, Salinsky MC. Pharmacologically induced changes in arousal: effects on behavioral and electrophysiologic measures of alertness and attention. Electroencephalogr and Clini Neurophysiol. 1995;95:359–371. doi: 10.1016/0013-4694(95)00124-h. [DOI] [PubMed] [Google Scholar]
- 40.Petajan JH. Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. 1996;39:432–441. doi: 10.1002/ana.410390405. [DOI] [PubMed] [Google Scholar]
- 41.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 42.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 43.Ware JF. SF-36 Health Survey: Manual interpretation Guide. Boston, Mass: The Health Institute; 1993.
- 44.Hui SS, Yuen PY. Validity of the modified back-saver sit-and-reach test: a comparison with other protocols. Med Sci Sports Exerc. 2000;32:1655–1659. doi: 10.1097/00005768-200009000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Kaye JA, Oken BS, Howieson DB, Howieson J, Holm LA, Dennison K. Neurologic evaluation of the optimally healthy oldest old. Arch Neurol. 1994;51:1205–1211. doi: 10.1001/archneur.1994.00540240049015. [DOI] [PubMed] [Google Scholar]
- 46.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 47.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 48.Yaffe K, Barnes D, Nevitt M, Lui L-Y, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 49.Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 50.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Archives of Neurology. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 51.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 52.Dustman RE, Emmerson R. Shearer D. Physical activity, age, and cognitive-neuropsychological function. J Aging Phy Act. 1994;2:143–181. [Google Scholar]
- 53.Gitlin LN, Lawton MP, Windsor-Landsberg LA. Kleban MH, Sands LP, Posner J. In search of psychological benefits: exercise in healthy older adults. J Aging and Health. 1992;4(2):174–192. [Google Scholar]
- 54.Hill RD, Storandt M, Malley M. The impact of long-term exercise training on psychological function in older adults. Journal of Gerontology. 1993;48:P12–P17. doi: 10.1093/geronj/48.1.p12. [DOI] [PubMed] [Google Scholar]
- 55.Blumenthal JA, Emery CF, Madden DJ, et al. Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. J Gerontol. 1989;44(5):M147–157. doi: 10.1093/geronj/44.5.m147. [DOI] [PubMed] [Google Scholar]
- 56.Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:14–15. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 57.Hawkins HL, Kramer AF, Capaldi D. Aging, exercise, and attention. Psychol Aging. 1992;7:643–653. doi: 10.1037//0882-7974.7.4.643. [DOI] [PubMed] [Google Scholar]
- 58.Kramer AF, Colcombe SJ, McAuley E, et al. Enhancing brain and cognitive function of older adults through fitness training. J Mol Neurosci. 2003;20:213–221. doi: 10.1385/JMN:20:3:213. [DOI] [PubMed] [Google Scholar]
- 59.Khatri P, Blumenthal JA, Babyak M, et al. Effects of exercise training on cognitive functioning among depressed older men and women. J Aging Phys Act. 2001;9:43–57. [Google Scholar]
- 60.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 61.Tse S-K, Bailey DM. T’ai Chi and postural control in the well elderly. Am J Occup Ther. 1992;46(4):295–300. doi: 10.5014/ajot.46.4.295. [DOI] [PubMed] [Google Scholar]
- 62.Li F, Fisher KJ, Harmer P. Irbe D, Tearse RG, Weimer C. Tai Chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J Am Geriat Soc. 2004;52:892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 63.Long BC. Aerobic conditioning and stress reduction: participation or conditioning? Hum Mov Sci. 1983;2:171–186. [Google Scholar]
- 64.Desharnais R, Jobin J, Cote C, Levesque L, Godin G. Aerobic exercise and the placebo effect: a controlled study. Psychosom Med. 1993;55:149–154. doi: 10.1097/00006842-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Oken BS. Placebo effect: clinical perspectives and potential mechanisms. In: Oken BS, ed. Complementary Therapies in Neurology: An Evidence-Based Approach. New York: Parthenon Publishing; 2004:199–230.
- 66.Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H. The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review. Health Technol Assess. 1999;3(3):1–96. [PubMed] [Google Scholar]
- 67.Canter PH. Ernst E. The cumulative effects of Transcendental Meditation on cognitive function—a systematic review of randomised controlled trials. Wien Klin Wochenschr. 2003;115:758–766. doi: 10.1007/BF03040500. [DOI] [PubMed] [Google Scholar]
- 68.Colcombe S. Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]


