Abstract
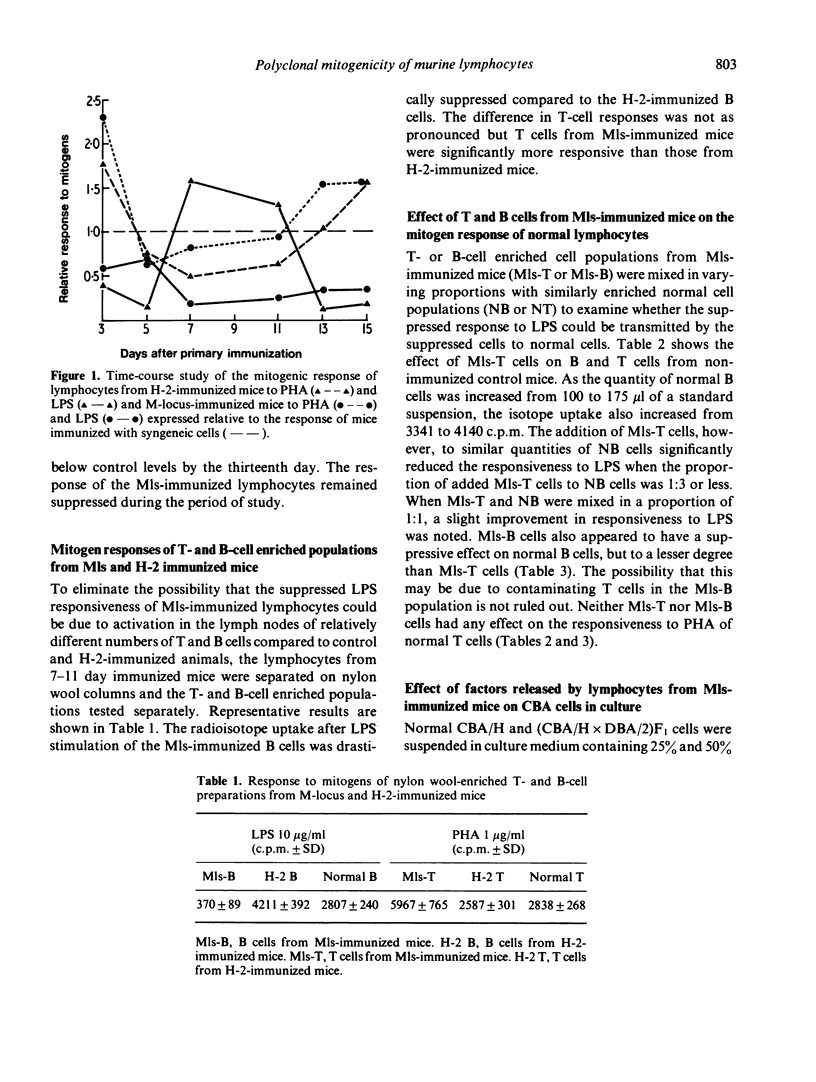
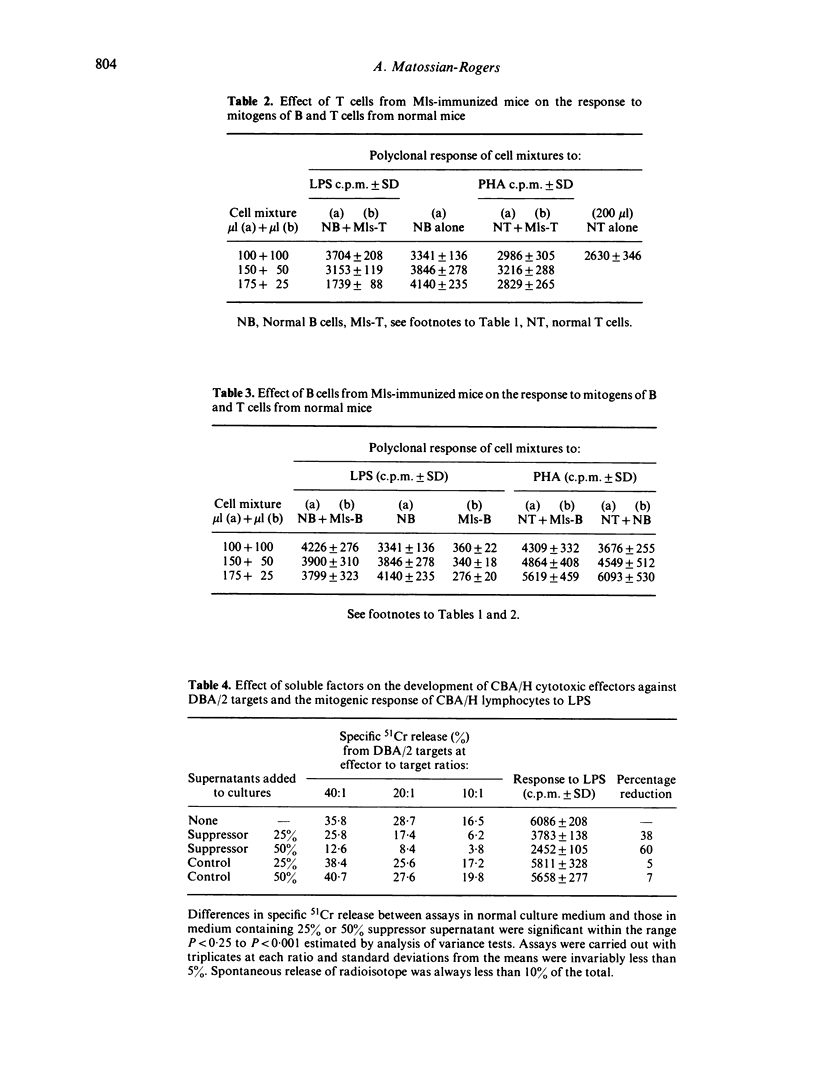
During the immune response to H-2 and Mls alloantigens, murine lymphocytes showed altered sensitivity to polyclonal mitogens. The reactivity to the T-cell mitogen PHA followed a similar pattern in both H-2 and Mls-immunized mice while the reactivity to the B-cell mitogen LPS was contrasting in the two groups. In the former group, the response exceeded control levels by the seventh day after immunization and then gradually dropped below control levels; the response of Mls-immunized lymphocytes dropped below control levels soon after immunization and remained so for the period of study. Nylon wool column-purified Mls-immunized B cells also showed a suppressed reactivity to LPS, while the T-enriched populations from Mls-immune mice when added to normal B cells lowered their LPS reactivity. Soluble factors derived from clutures of Mls-immune lymphocytes had a suppressive effect on normal B cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Callard R. E., Basten A. Immune function in aged mice. I. T-cell responsiveness using phytohaemagglutinin as a functional probe. Cell Immunol. 1977 Jun 1;31(1):13–25. doi: 10.1016/0008-8749(77)90002-8. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Moller G., Gronowicz E. Genetical control of B-cell responses. IV. Inheritance of the unresponsiveness to lipopolysaccharides. J Exp Med. 1975 Jul 1;142(1):253–258. doi: 10.1084/jem.142.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debré P., Kapp J. A., Benacerraf B. Genetic control of specific immune suppression. I. Experimental conditions for the stimulation of suppressor cells by the copolymer L-glutamic acid50-L-tyrosine50 (GT) in nonresponder BALB/c mice. J Exp Med. 1975 Dec 1;142(6):1436–1446. doi: 10.1084/jem.142.6.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C., Möller G. Induction of immunological tolerance requires that the B cells can respond to the polyclonal B-cell-activating properties of the thymus-independent antigens. J Exp Med. 1977 Jul 1;146(1):308–312. doi: 10.1084/jem.146.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festenstein H., Abbasi K., Sachs J. A., Oliver R. T. Serologically undetectable immune responses in transplantation. Transplant Proc. 1972 Jun;4(2):219–222. [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Matossian-Rogers A., Festenstein H. Generation of suppressor cells in mice immunized with M locus-incompatible lymphocytes. Transplantation. 1977 Apr;23(4):316–321. doi: 10.1097/00007890-197704000-00004. [DOI] [PubMed] [Google Scholar]
- Matossian-Rogers A., Festenstein H. Modification of murine T-cell cytotoxicity by preimmunization with M locus and H-2 incompatibilities. J Exp Med. 1976 Feb 1;143(2):456–461. doi: 10.1084/jem.143.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Mathieson B. J., Campbell P. S. Ly phenotype and mechanism of action of mouse neonatal suppressor T cells. J Exp Med. 1977 Jul 1;146(1):59–73. doi: 10.1084/jem.146.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness D. B., Smith S., Talcott J. A., Grumet F. C. T cell requirements for the expression of the lipopolysaccharide adjuvant effect in vivo: evidence for a T cell-dependent and a T cell-independent mode of action. Eur J Immunol. 1976 Sep;6(9):650–654. doi: 10.1002/eji.1830060911. [DOI] [PubMed] [Google Scholar]
- Newburger P. E., Hamaoka T., Katz D. H. Potentiation of helper T cell function in IgE antibody responses by bacterial lipolysaccharide (LPS). J Immunol. 1974 Sep;113(3):824–829. [PubMed] [Google Scholar]
- Norcross M. A., Smith R. T. Regulation of B-cell proliferative responses to lipopolysaccharide by a subclass of thymus T cells. J Exp Med. 1977 May 1;145(5):1299–1315. doi: 10.1084/jem.145.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röllinghoff M., Wagner H. The allogeneic effect: M-locus differences substitute for differences in the H-2 major histocompatibility complex. J Immunol. 1975 Apr;114(4):1329–1332. [PubMed] [Google Scholar]
- Sachs J. A., Huber B., Peña-Martinez J., Festenstein H. Genetic studies and effect on skin allograft survival of DBA-2 DAG, Ly, and M-locus antigens. Transplant Proc. 1973 Dec;5(4):1385–1387. [PubMed] [Google Scholar]
- Shinohara N., Kern M. Differentiation of lymphoid cells: B cell as a direct target and T cell as a regulator in lipopolysaccharide-enhanced induction of immunoglobulin production. J Immunol. 1976 Jun;116(6):1607–1612. [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga M., Yoshinaga A., Waksman B. H. Regulation of lymphocyte responses in vitro. I. Regulatory effect of macrophages and thymus-dependent (T) cells on the response of thymus-independent (B) lymphocytes to endotoxin. J Exp Med. 1972 Oct 1;136(4):956–961. doi: 10.1084/jem.136.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]


