Abstract
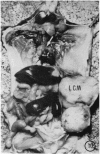
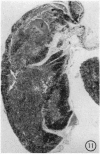
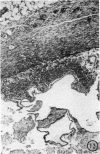
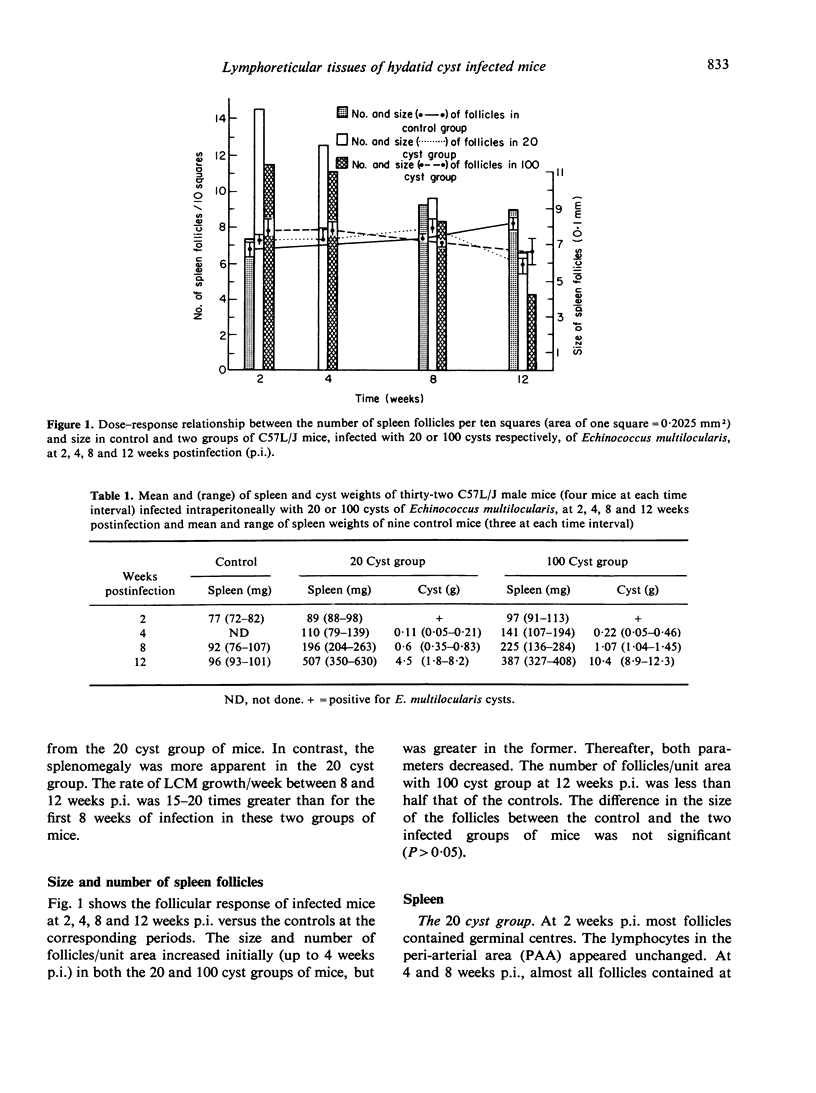
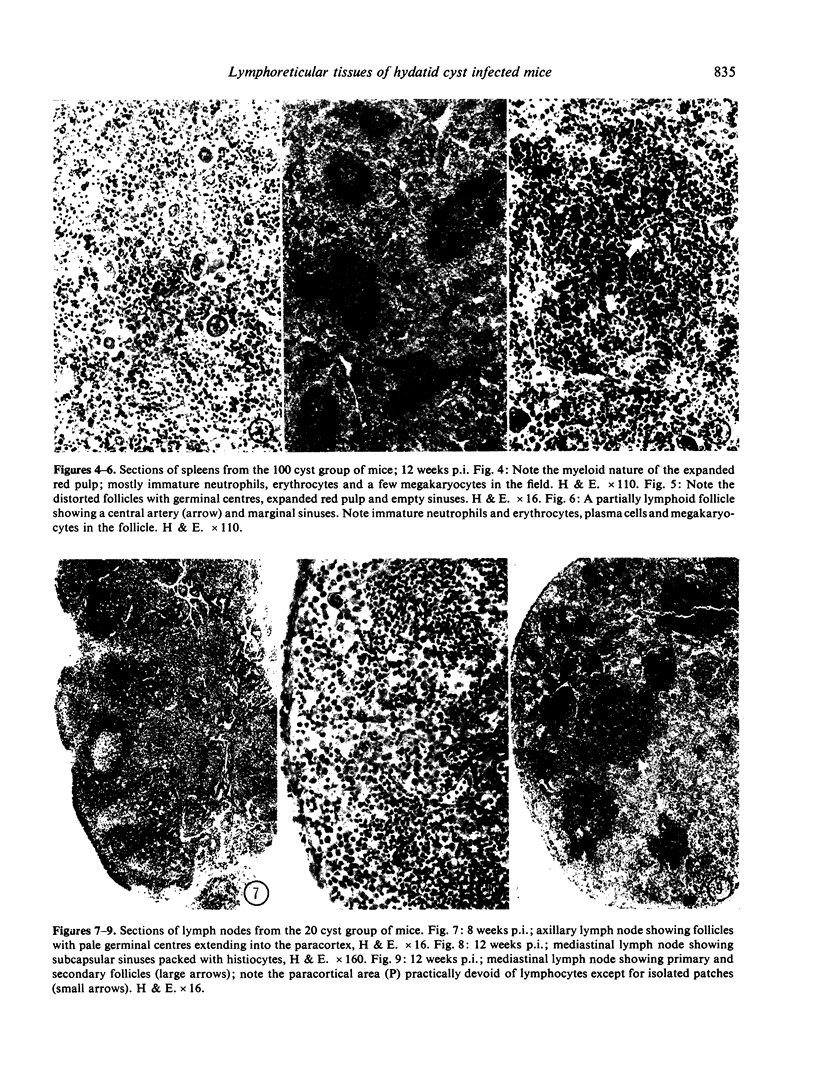
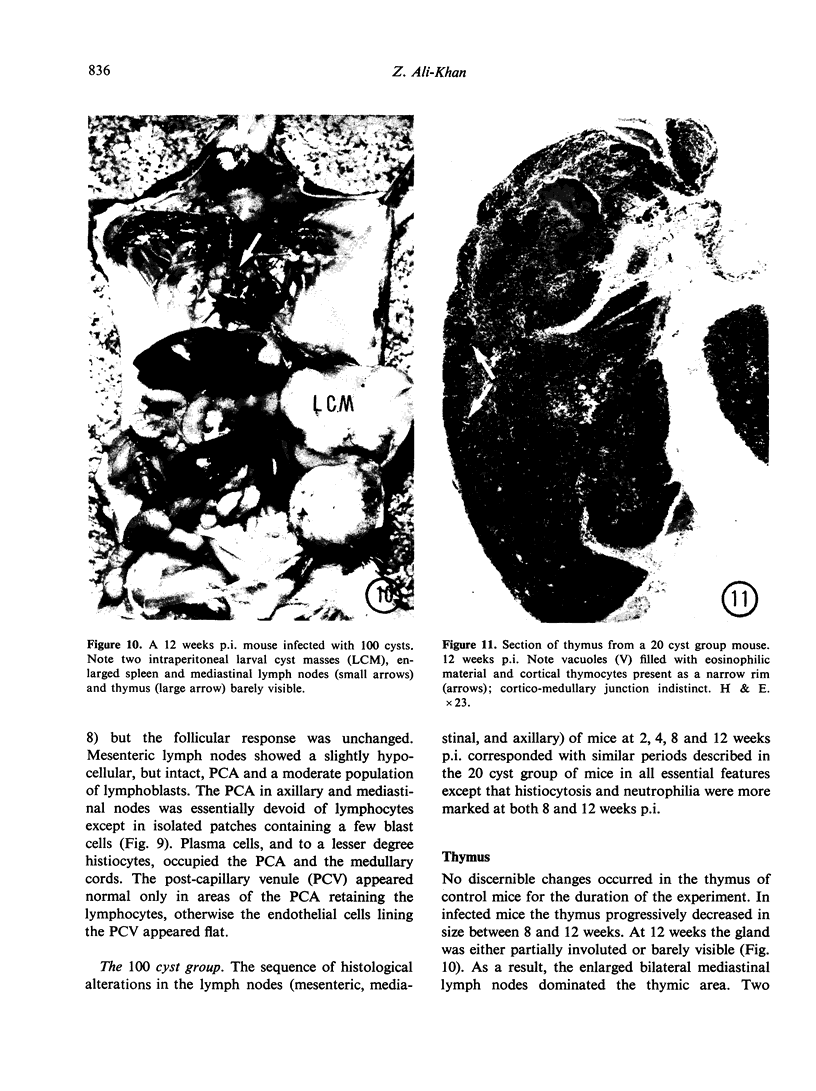
The pathology of the spleen, lymph nodes and thymus of C57L/J mice, infected intraperitoneally with 20 and 100 cysts of Echinococcus multilocularis is described at 2, 4, 8 and 12 weeks postinfection (p.i.). For the first 8 weeks, growth of the larval cyst mass (LCM) was slow and blastogenesis in T-dependent areas of both spleen and lymph nodes was moderate whereas in B-cell compartments it was intense. A rapid growth of the LCM between 8 and 12 weeks p.i., 15-20 times greater than for the first 8 weeks, was associated with depletion of lymphocytes in thymus dependent area (TDA) of both spleen and lymph nodes, gross expansion of the red pulp with extramedullary haemopoiesis, partial atrophy of spleen follicles, but not those of the lymph node, and involution of the thymus. At 12 weeks p.i. the TDA had mainly plasma cells and histiocytes among occasional lymphocytes and blast cells; germinal centre activity and plasmacytosis persisted in B-cell areas. Morphological aspects of these changes are discussed in relation to the invasiveness and proliferation of the LCM during the course of infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali-Khan Z. Host-parasite relationship in echinococcosis. I. Parasite biomass and antibody response in three strains of inbred mice against graded doses of Echinococcus multilocularis cysts. J Parasitol. 1974 Apr;60(2):231–235. [PubMed] [Google Scholar]
- Ali-Khan Z. Host-parasite relationship in echinococcosis. II. Cyst weight, hematologic alterations, and gross changes in the spleen and lymph nodes of C57L mice against graded doses of Echinococcus multilocularis cysts. J Parasitol. 1974 Apr;60(2):236–242. [PubMed] [Google Scholar]
- CONGDON C. C. Effect of injection of foreign bone marrow on the lymphatic tissues of normal mice. J Natl Cancer Inst. 1962 Feb;28:305–329. [PubMed] [Google Scholar]
- CONGDON C. C., MAKINODAN T. Splenic white pulp alteration after antigen injection: relation to time of serum antibody production. Am J Pathol. 1961 Dec;39:697–709. [PMC free article] [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V., Dietrich F. M. The morphology of immune reactions in normal, thymectomized and reconstituted mice. 3. Response to bacterial antigens: salmonellar flagellar antigen and pneumococcal plysaccharide. Immunology. 1970 Dec;19(6):945–957. [PMC free article] [PubMed] [Google Scholar]
- Feldman J. D. Immunological enhancement: a study of blocking antibodies. Adv Immunol. 1972;15:167–214. doi: 10.1016/s0065-2776(08)60685-9. [DOI] [PubMed] [Google Scholar]
- HANNA M. G., Jr GERMINAL CENTER CHANGES AND PLASMA CELL REACTION DURING THE PRIMARY IMMUNE RESPONSE. Int Arch Allergy Appl Immunol. 1965;26:230–251. doi: 10.1159/000229574. [DOI] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D. BASIS FOR IMMUNITY TO TYPHOID IN MICE AND THE QUESTION OF "CELLULAR IMMUNITY". Bacteriol Rev. 1963 Dec;27:391–404. doi: 10.1128/br.27.4.391-404.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANKAU S. K. Studies on Echinococcus alveolaris (Klemm, 1883), from St. Lawrence Island, Alaska. III. The histopathology caused by the infection of E. alveolaris in white mice. Am J Trop Med Hyg. 1956 Sep;5(5):872–880. doi: 10.4269/ajtmh.1956.5.872. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Auclair D. J., Lagrange P. H. Immunopotentiation with BCG. I. Immune response to different strains and preparations. J Natl Cancer Inst. 1973 Nov;51(5):1655–1667. doi: 10.1093/jnci/51.5.1655. [DOI] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. II. The role of the T and B lymphocytes. Immunology. 1974 Nov;27(5):825–840. [PMC free article] [PubMed] [Google Scholar]
- North R. J., Mackaness G. B., Elliott R. W. The histogenesis of immunologically committed lymphocytes. Cell Immunol. 1972 Apr;3(4):680–694. doi: 10.1016/0008-8749(72)90130-x. [DOI] [PubMed] [Google Scholar]
- Olson I. A., Hunt A. C., Verrier-Jones J. Competition between two delayed hypersensitivity antigens for the same draining lymph-node: the histiocytic response compared with sinus histiocytosis. J Pathol. 1971 Feb;103(2):107–112. doi: 10.1002/path.1711030205. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., De Sousa M. Thymus-dependent and thymus-independent populations: origin, migratory patterns and lifespan. Clin Exp Immunol. 1971 May;8(5):663–684. [PMC free article] [PubMed] [Google Scholar]
- Preston P. M., Carter R. L., Leuchars E., Davies A. J., Dumonde D. C. Experimental cutaneous leishmaniasis. 3. Effects of thymectomy on the course of infection of CBA mice with Leishmania tropica. Clin Exp Immunol. 1972 Feb;10(2):337–357. [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Gaugas J. M., Rees R. J., Allison A. C. Immune responses in mice with murine leprosy. Clin Exp Immunol. 1970 Jan;6(1):117–124. [PMC free article] [PubMed] [Google Scholar]
- RAUSCH R. Studies on the helminth fauna of Alaska. XX. The histogenesis of the alveolar larva of Echinococcus species. J Infect Dis. 1954 Mar-Apr;94(2):178–186. doi: 10.1093/infdis/94.2.178. [DOI] [PubMed] [Google Scholar]
- Rogers R., Denham D. A., Nelson G. S., Guy F., Ponnudurai T. Studies with Brugia pahangi. III: Histological changes in the affected lymph nodes of infected cats. Ann Trop Med Parasitol. 1975 Mar;69(1):77–84. [PubMed] [Google Scholar]
- TALIAFERRO W. H. Functions of the spleen in immunity; presidential address. Am J Trop Med Hyg. 1956 May;5(3):391–410. doi: 10.4269/ajtmh.1956.5.391. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Waters M. F. Immunological significance of changes in lymph nodes across the leprosy spectrum. Clin Exp Immunol. 1971 Mar;8(3):363–376. [PMC free article] [PubMed] [Google Scholar]
- Woodruff A. W. Mechanisms involved in anaemia associated with infection and splenomegaly in the tropics. Trans R Soc Trop Med Hyg. 1973;67(3):313–328. doi: 10.1016/0035-9203(73)90107-7. [DOI] [PubMed] [Google Scholar]