Abstract
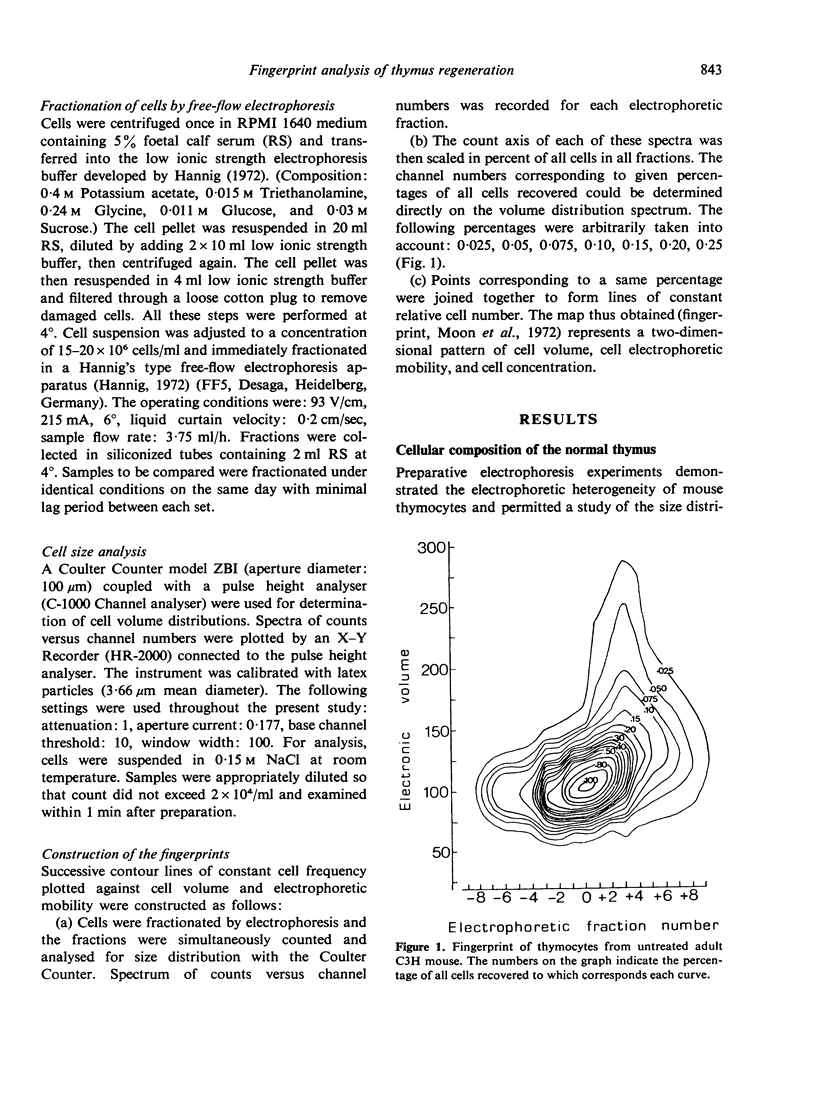
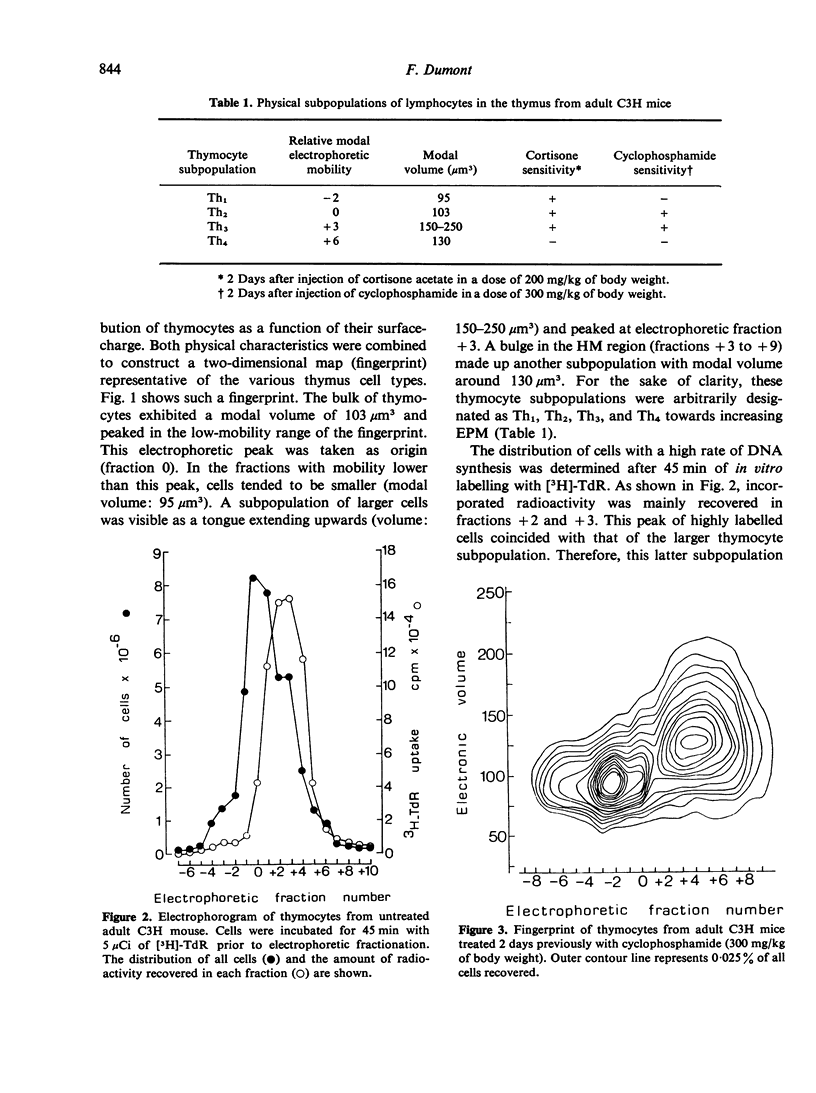
Thymocytes from adult C3H mice were fractionated on the basis of electrophoretic mobility (EPM) differences by free-flow electrophoresis and the fractions obtained were analysed for size distribution with a Coulter Counter. The data were combined in the form of contour maps (fingerprints) representative of the various physical types of thymocytes. Four thymocyte subpopulations with distinct physical properties were thus characterized and were further shown to differ in their sensitivity to immunosuppressive drugs and in their proliferation rate. Th1 cells possess the slowest EPM and smallest volume (95 μm3), are sensitive to cortisone (C) but resistant to cyclophosphamide (Cy) and do not incorporate 3[H]-thymidine (TdR) in vitro. Th2 cells possess higher EPM and slightly larger volume (103 μm3) and are sensitive to both C and Cy. Th3 cells are of still higher EPM, exhibit the largest volume (150–250 μm3) of all thymocytes, are sensitive to C and Cy and rapidly incorporate [3H]-TdR in vitro. Th4 cells are endowed with fastest EPM and a modal volume of 130 μm3 and are resistant to both C and Cy.
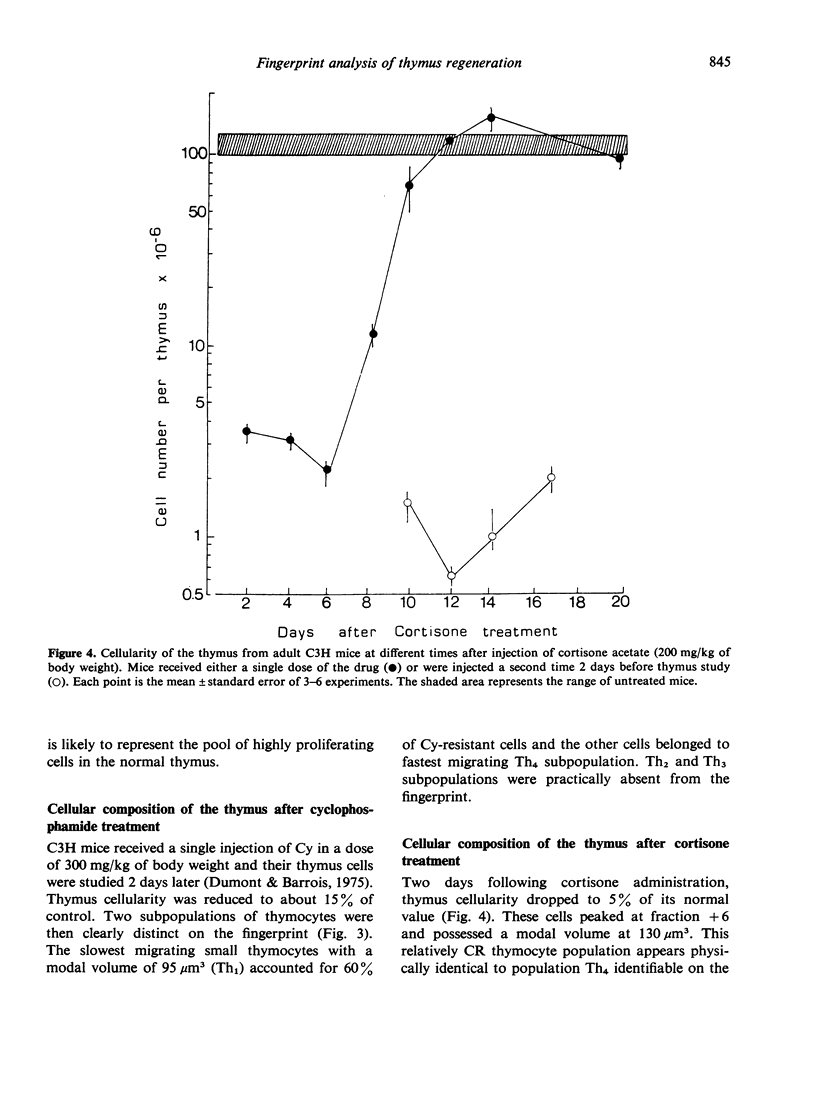
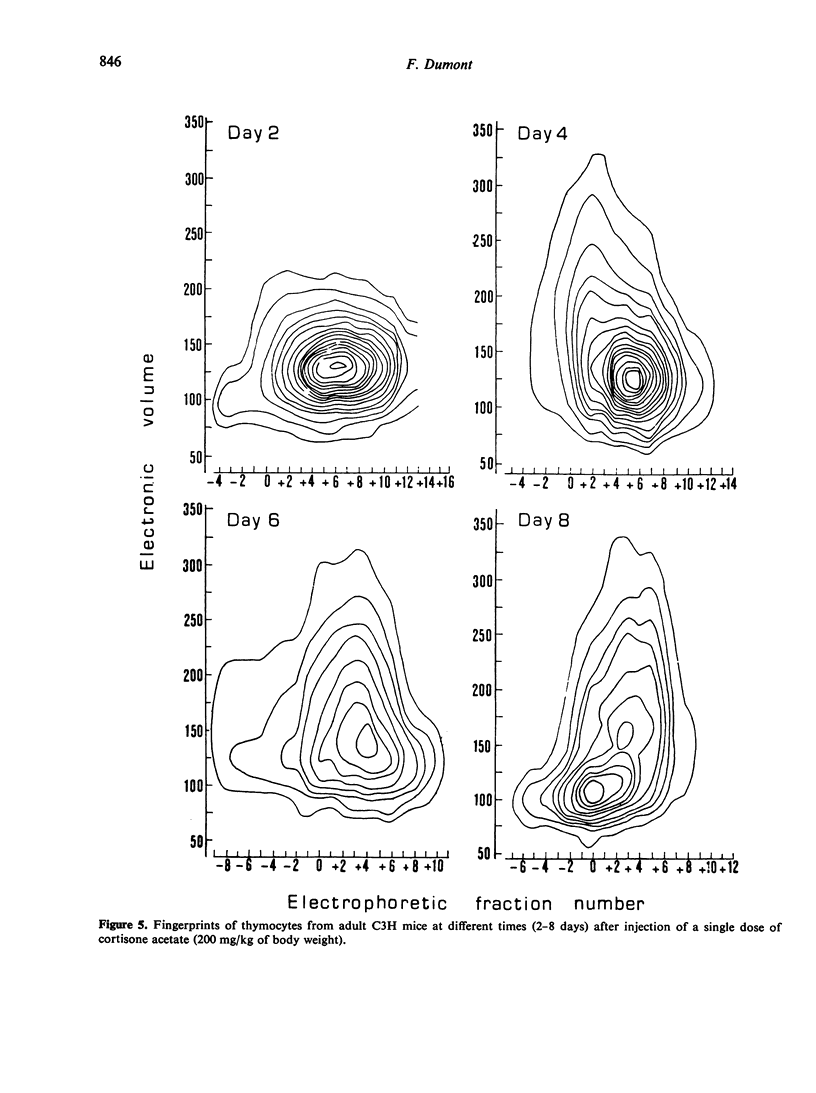
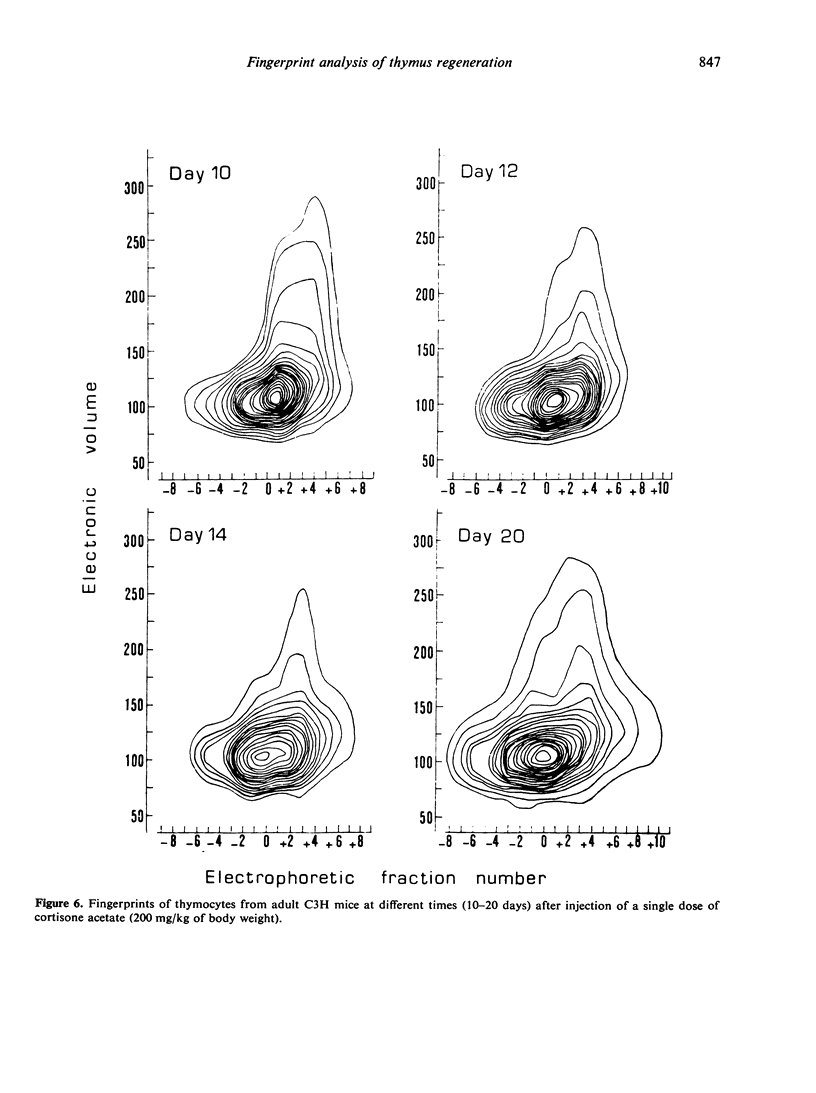
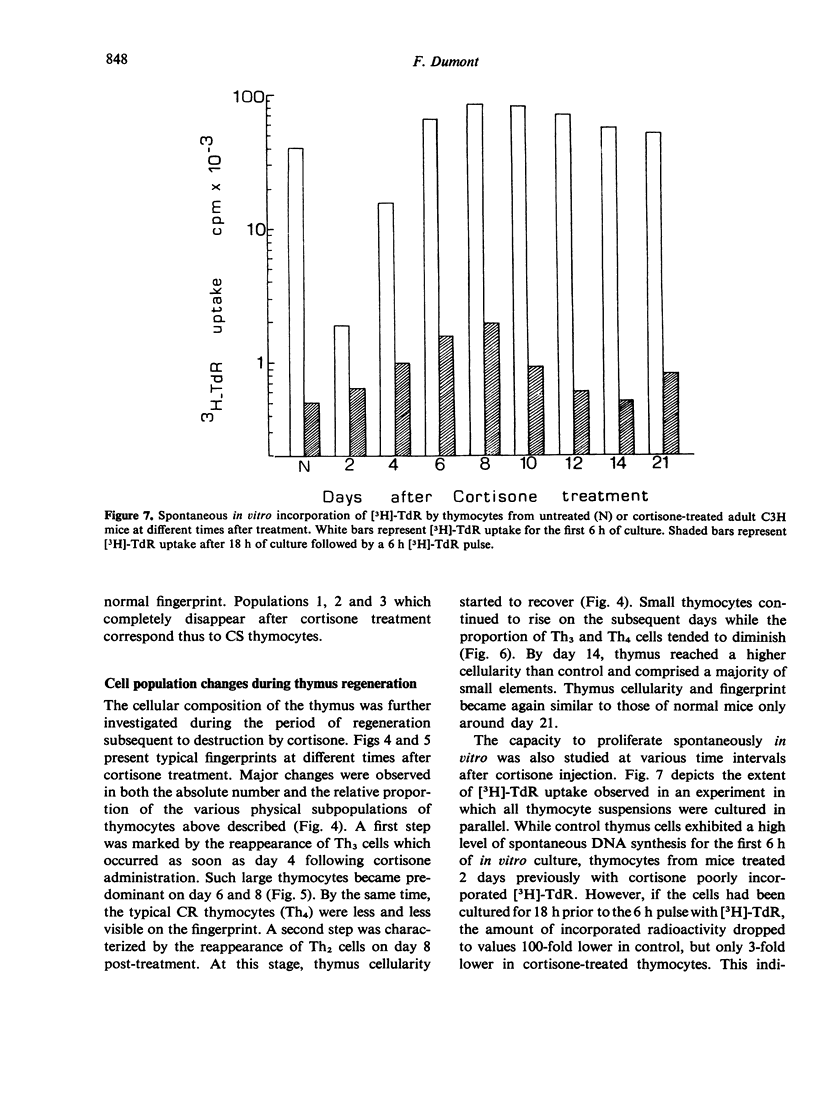
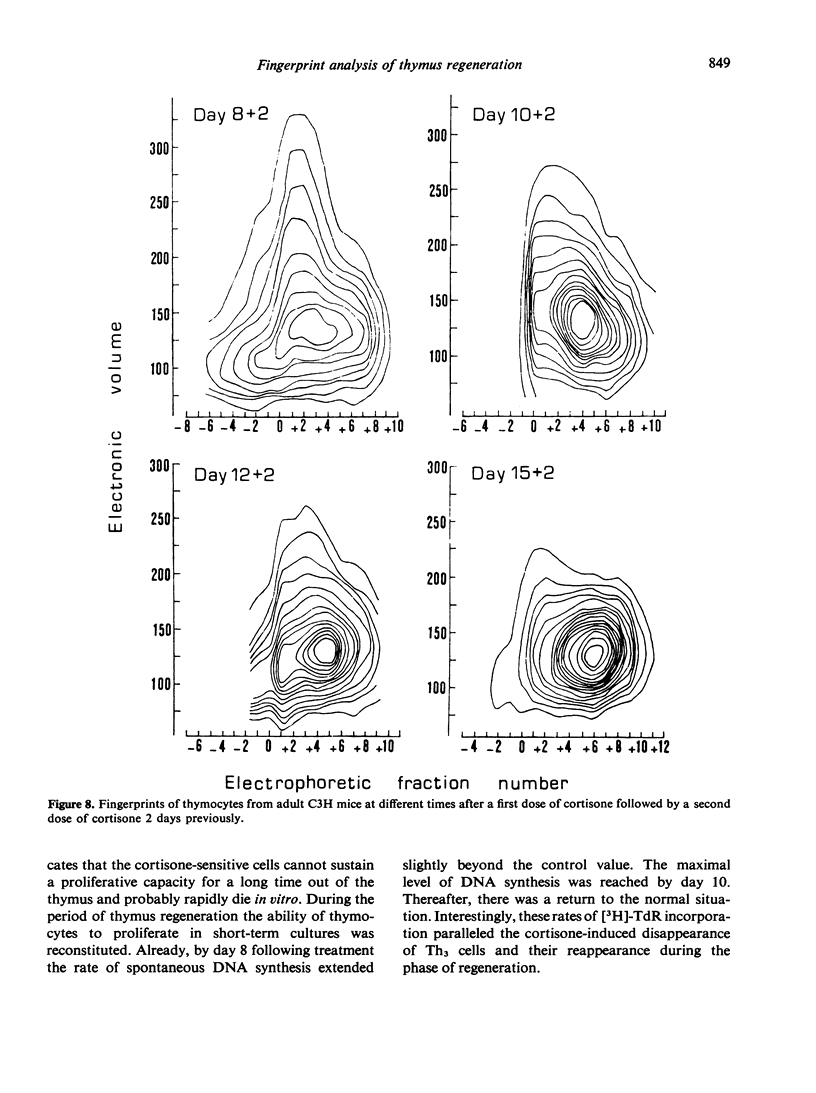
The regeneration and fate of these subpopulations were investigated during the period subsequent to cortisone injection. Th3 cells were the first to reappear on day 4–6 following treatment. Thereafter, Th2 and Th1 cells began to rise again and eventually reached levels higher than control by day 12–14 post-treatment. By the same time, Th4 cells, which escaped cortisone lympholytic action became less and less visible on the fingerprint. In fact, administration of a second dose of cortisone by day 8–10 after the first treatment revealed a depletion as well as a physical modification of the C-resistant cell pool. Typical Th4 cells were found again on day 15 after the first cortisone injection. It was only around day 20 that thymus became normally repopulated.
Taken together, these observations indicate that Th3 cells may act as precursors for all 3 other thymocyte subpopulations and that during thymus reconstitution Th2 and Th1 cells are produced at a faster rate than are Th4 cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomgren H., Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970 Nov;1(5):545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Evidence for a small pool of immunocompetent cells in the mouse thymus. Exp Cell Res. 1969 Oct;57(2):185–192. doi: 10.1016/0014-4827(69)90140-2. [DOI] [PubMed] [Google Scholar]
- Bryant B. J. Renewal and fate in the mammalian thymus: mechanisms and inferences of thymocytokinetics. Eur J Immunol. 1972 Feb;2(1):38–45. doi: 10.1002/eji.1830020109. [DOI] [PubMed] [Google Scholar]
- DeWys W. D., Goldin A., Man, El N. Hematopoietic recovery after large doses of cyclophosphamide: correlation of proliferative state with sensitivity. Cancer Res. 1970 Jun;30(6):1692–1697. [PubMed] [Google Scholar]
- Droege W., Zucker R., Jauker U. Cellular composition of the mouse thymus: developmental changes and the effect of hydrocortisone. Cell Immunol. 1974 May;12(2):173–185. doi: 10.1016/0008-8749(74)90070-7. [DOI] [PubMed] [Google Scholar]
- Droege W., Zucker R. Lymphocyte subpopulations in the thymus. Transplant Rev. 1975;25:3–25. doi: 10.1111/j.1600-065x.1975.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Dumont F., Barrois R. Effect of treatment with cyclophosphamide on the electrophoretic mobility and mitogen responsiveness of mouse thymus cells. Biomedicine. 1975 Nov 10;23(9):391–395. [PubMed] [Google Scholar]
- Dumont F. Electrophoretic analysis of cell subpopulations in the mouse thymus as a function of age. Immunology. 1974 May;26(5):1051–1057. [PMC free article] [PubMed] [Google Scholar]
- Dumont F., Robert F. Dose-related effect of hydrocortisone treatment on the electrokinetic properties and mitogen responsiveness of mouse thymocytes. Int Arch Allergy Appl Immunol. 1976;51(4):482–495. doi: 10.1159/000231622. [DOI] [PubMed] [Google Scholar]
- Dumont F., Sabolović D. Heterogeneity of the hydrocortisone-resistant cell subpopulation in the mouse thymus. Biomedicine. 1973 Jun 20;19(6):257–260. [PubMed] [Google Scholar]
- Fathman C. G., Small M., Herzenberg L. A., Weissman I. L. Thymus cell maturation. II. Differentiation of three "mature" subclasses in vivo. Cell Immunol. 1975 Jan;15(1):109–128. doi: 10.1016/0008-8749(75)90169-0. [DOI] [PubMed] [Google Scholar]
- ISHIDATE M., METCALF D. THE PATTERN OF LYMPHOPOIESIS IN THE MOUSE THYMUS AFTER CORTISONE ADMINISTRATION OR ADRENALECTOMY. Aust J Exp Biol Med Sci. 1963 Dec;41:637–649. doi: 10.1038/icb.1963.53. [DOI] [PubMed] [Google Scholar]
- Jacobsson H., Blomgren H. Changes of the PHA-responding pool of cells in the thymus after cortisone or x-ray treatment of mice. Evidence for an invese relation between the production of cortical and medullary thymocytes. Cell Immunol. 1972 May;4(1):93–105. doi: 10.1016/0008-8749(72)90008-1. [DOI] [PubMed] [Google Scholar]
- Jacobsson H., Blomgren H. Evidence of different cell populations in the mouse thymus releasing and responding to mitogenic factor. Scand J Immunol. 1975;4(8):791–799. doi: 10.1111/j.1365-3083.1975.tb03719.x. [DOI] [PubMed] [Google Scholar]
- Kadish J. L., Basch R. S. Thymic regeneration after lethal irradiation evidence for an intra-thymic radioresistant T cell precursor. J Immunol. 1975 Jan;114(1 Pt 2):452–458. [PubMed] [Google Scholar]
- Moon R., Phillips R. A., Miller R. G. Sedimentation and volume analysis of human bone marrow. Ser Haematol. 1972;5(2):163–178. [PubMed] [Google Scholar]
- Sabolović D., Dumont F. Separation and characterization of cell subpopulations in the thymus. Immunology. 1973 Apr;24(4):601–606. [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. Antigens of the thymus. Prog Allergy. 1972;16:214–299. doi: 10.1159/000313173. [DOI] [PubMed] [Google Scholar]
- Schlesinger M., Israël E. The recovery of spleen-seeking and lymph-node-seeking thymus subpopulations following cortisol administration. Cell Immunol. 1975 Jul;18(1):144–151. doi: 10.1016/0008-8749(75)90043-x. [DOI] [PubMed] [Google Scholar]
- Shortman K., Jackson H. The differentiation of T lymphocytes. I. Proliferation kinetics and interrelationships of subpopulations of mouse thymus cells. Cell Immunol. 1974 May;12(2):230–246. doi: 10.1016/0008-8749(74)90075-6. [DOI] [PubMed] [Google Scholar]
- Wioland M., Sabolovic D., Burg C. Electrophoretic mobilities of T and B cells. Nat New Biol. 1972 Jun 28;237(78):274–276. doi: 10.1038/newbio237274a0. [DOI] [PubMed] [Google Scholar]
- Zeiller K., Pascher G., Wagner G., Liebich H. G., Holzberg E., Hannig K. Distinct subpopulations of thymus-dependent lymphocytes. Tracing of the differentiation pathway of T cells by use of preparatively electrophoretically separated mouse lymphocytes. Immunology. 1974 May;26(5):995–1012. [PMC free article] [PubMed] [Google Scholar]


