Abstract
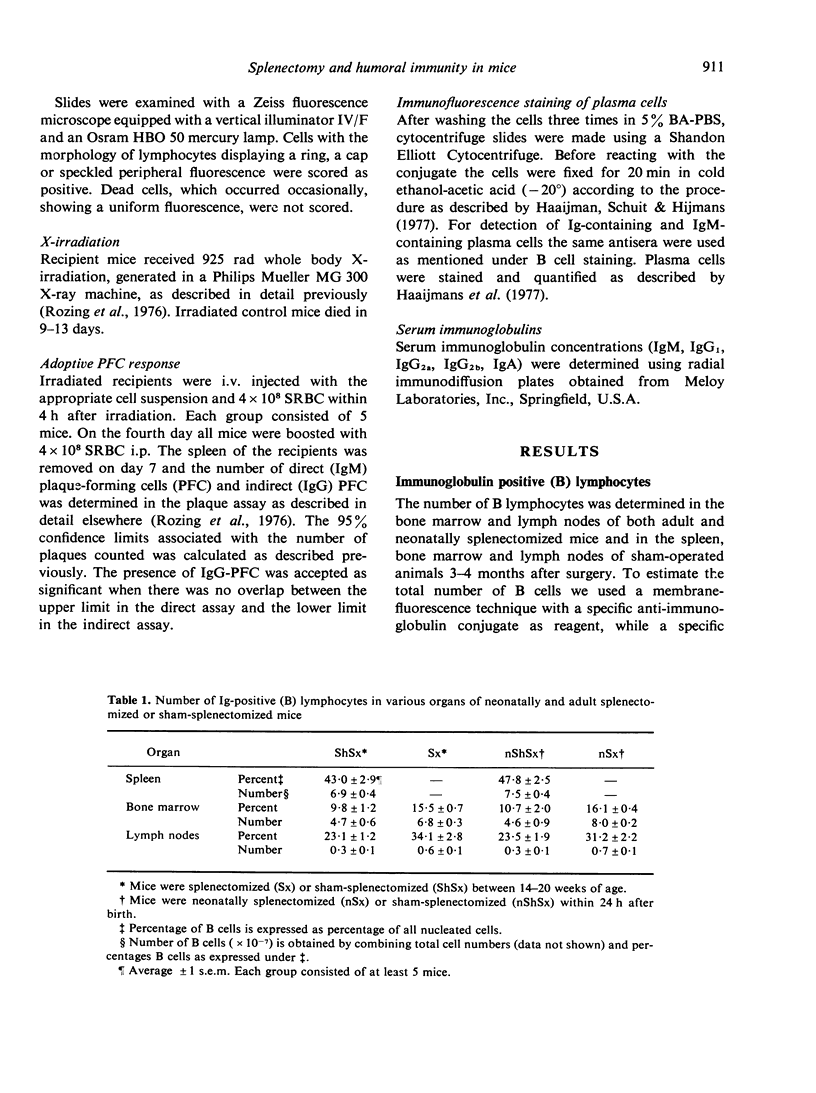
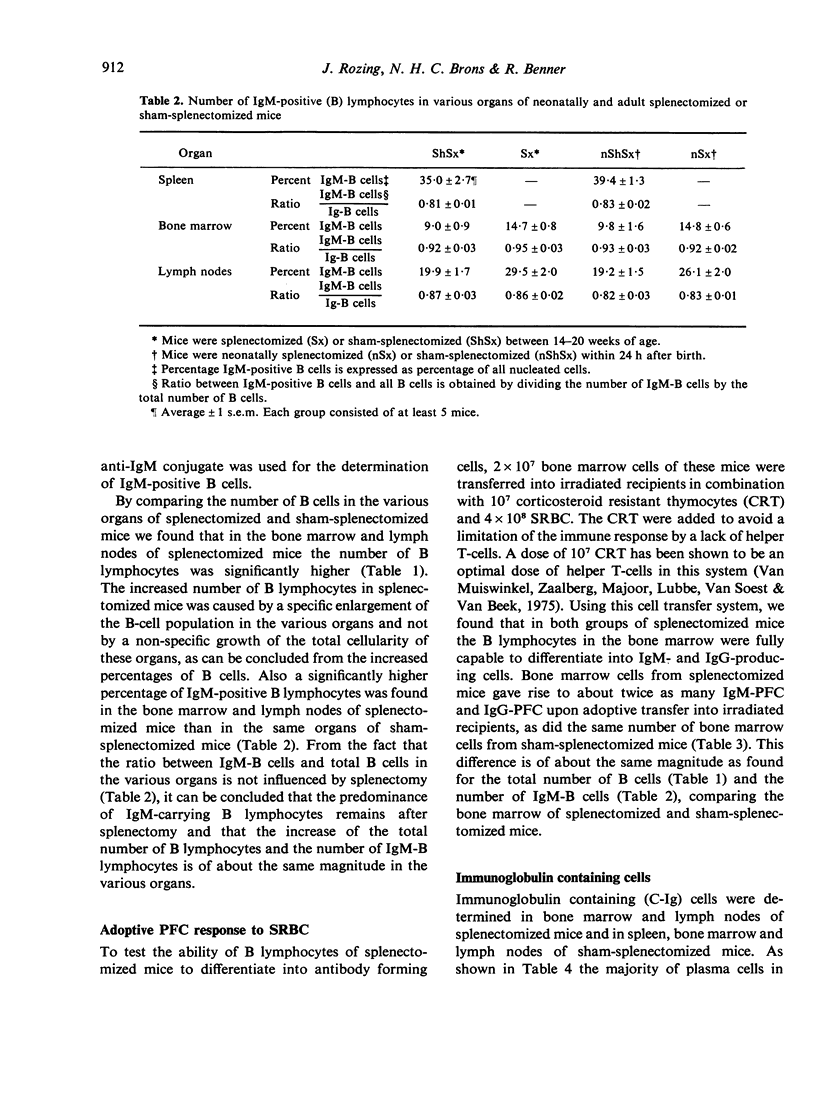
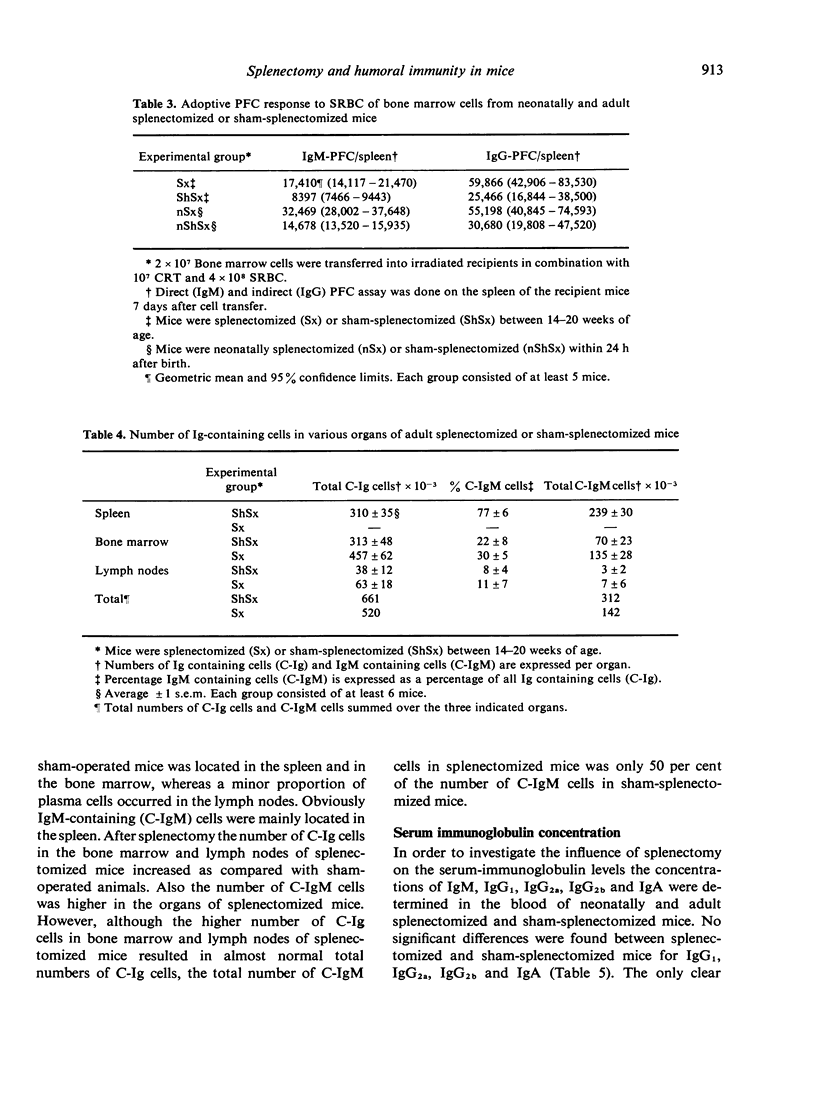
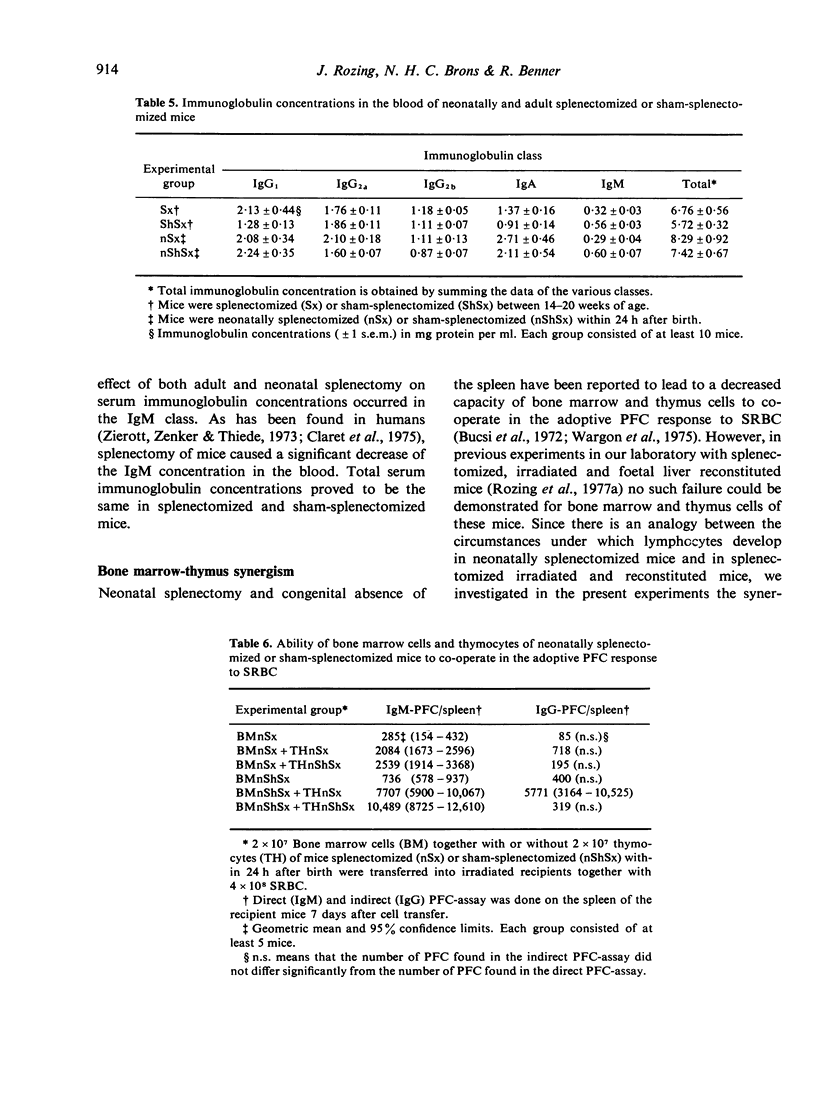
Experiments were performed to investigate the influence of neonatal and adult splenectomy on humoral immunity in mice. In the bone marrow and lymph nodes of both groups of splenectomized mice the number of immunoglobulin (Ig)-positive (B) lymphocytes was significantly higher than in sham-operated mice. These higher numbers of B cells probably reflect a compensation for the absence of the B cell population of the spleen. Hardly any quantitative differences in the serum immunoglobulins were found between splenectomized and sham-splenectomized mice. Only for the IgM class was a significantly lower concentration found in the serum of splenectomized animals. This low concentration of IgM in the blood of splenectomized mice was caused by a failure of the remaining organs to compensate completely for the removal of the quantitatively important population of IgM-producing plasma cells in the spleen. Nevertheless, the number of precursors of IgM-producing plasma cells in bone marrow and lymph nodes and their ability to differentiate into IgM-producing plasma cells was not diminished by splenectomy. Probably the spleen provides a highly efficient environment for the differentiation into IgM-producing plasma cells.
By investigating the synergistic ability of bone marrow cells and thymus cells from neonatally splenectomized mice it was found that these cells were fully capable of co-operating in the adoptive plaque-forming cell response to sheep red blood cells (SRBC).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUERBACH R. Developmental studies of mouse thymus and spleen. Natl Cancer Inst Monogr. 1963 Mar;11:23–33. [PubMed] [Google Scholar]
- Basten A., Miller J. F., Warner N. L., Pye J. Specific inactivation of thymus-derived (T) and non-thymus-derived (B) lymphocytes by 125I-labelled antigen. Nat New Biol. 1971 May 26;231(21):104–106. doi: 10.1038/newbio231104a0. [DOI] [PubMed] [Google Scholar]
- Benner R., van Oudenaren A. Antibody formation in mouse bone marrow. IV. The influence of splenectomy on the bone marrow plaque-forming cell response to sheep red blood cells. Cell Immunol. 1975 Oct;19(2):167–182. doi: 10.1016/0008-8749(75)90201-4. [DOI] [PubMed] [Google Scholar]
- Bucsi R. A., Borek F., Battisto J. R. Splenic replenishment of synergistic ability to bone marrow and thymic cells of neonatally splenectomized CBA mice. J Exp Med. 1972 Oct 1;136(4):761–768. doi: 10.1084/jem.136.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R., Marsh J. C., Cartwright G. E., Wintrobe M. M. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968 Aug;215(2):353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- Claret I., Morales L., Montaner A. Immunological studies in the postsplenectomy syndrome. J Pediatr Surg. 1975 Feb;10(1):59–64. doi: 10.1016/s0022-3468(75)80009-1. [DOI] [PubMed] [Google Scholar]
- HALLER J. A., Jr THE EFFECT OF NEONATAL SPLENECTOMY ON MORTALITY FROM RUNT DISEASE IN MICE. Transplantation. 1964 Mar;2:287–291. doi: 10.1097/00007890-196403000-00016. [DOI] [PubMed] [Google Scholar]
- Haaijman J. J., Schuit H. R., Hijmans W. Immunoglobulin-containing cells in different lymphoid organs of the CBA mouse during its life-span. Immunology. 1977 Apr;32(4):427–434. [PMC free article] [PubMed] [Google Scholar]
- Haller J. A., Jr Role of the spleen in experimental neonatal infections and transplantation. J Pediatr Surg. 1970 Apr;5(2):172–175. doi: 10.1016/0022-3468(70)90273-3. [DOI] [PubMed] [Google Scholar]
- Kalpaktsoglou P. K., Good R. A. Mortality and five classes of mouse immunoglobulins in early splenectomy. Int Arch Allergy Appl Immunol. 1973;44(5):697–705. doi: 10.1159/000230972. [DOI] [PubMed] [Google Scholar]
- Kevy S. V., Tefft M., Vawier G. F., Rosen F. S. Hereditary splenic hypoplasia. Pediatrics. 1968 Nov;42(5):752–757. [PubMed] [Google Scholar]
- Osmond D. G. Formation and maturation of bone marrow lymphocytes. J Reticuloendothel Soc. 1975 Feb;17(2):99–114. [PubMed] [Google Scholar]
- Osmond D. G., Nossal G. J. Differentiation of lymphocytes in mouse bone marrow. I. Quantitative radioautographic studies of antiglobulin binding by lymphocytes in bone marrow and lymphoid tissues. Cell Immunol. 1974 Jul;13(1):117–131. doi: 10.1016/0008-8749(74)90232-9. [DOI] [PubMed] [Google Scholar]
- Pedersen B., Videbaek A. On the late effects of removal of the normal spleen. A follow-up study of 40 persons. Acta Chir Scand. 1966 Jan-Feb;131(1):89–98. [PubMed] [Google Scholar]
- Pierce J. C., Hume D. M. The effect of splenectomy on the survival of first and second renal homotransplants in man. Surg Gynecol Obstet. 1968 Dec;127(6):1300–1306. [PubMed] [Google Scholar]
- ROWLEY D. A. The formation of circulating antibody in the splenectomized human being following intravenous injection of heterologous erythrocytes. J Immunol. 1950 Nov;65(5):515–521. [PubMed] [Google Scholar]
- Rozing J., Brons N. H., Benner R. B-Lymphocyte differentiation in lethally irradiated and reconstituted mice. II. Recovery of humoral immune responsiveness. Cell Immunol. 1977 Mar 1;29(1):37–53. doi: 10.1016/0008-8749(77)90273-8. [DOI] [PubMed] [Google Scholar]
- Rozing J., Brons N. H., Benner R. B-lymphocyte differentiation in lethally irradiated and reconstituted mice. III. The influence of splenectomy on the recovery of the B-cell populations. Cell Immunol. 1977 Jun 15;31(2):340–348. doi: 10.1016/0008-8749(77)90035-1. [DOI] [PubMed] [Google Scholar]
- Rozing J., Buurman W. A., Benner R. B lymphocyte differentiation in lethally irradiated and reconstituted mice. I. The effect of Strontium-89 induced bone marrow aplasia on the recovery of the B cell compartment in the spleen. Cell Immunol. 1976 Jun 1;24(1):79–89. doi: 10.1016/0008-8749(76)90133-7. [DOI] [PubMed] [Google Scholar]
- Shinefield H. R., Steinberg C. R., Kaye D. Effect of splenectomy on the susceptibility of mice inoculated with Diplococcus pneumoniae. J Exp Med. 1966 May 1;123(5):777–794. doi: 10.1084/jem.123.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19(0):259–294. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]
- Stocker J. W., Osmond D. G., Nossal G. J. Differentiation of lymphocytes in the mouse bone marrow. III. The adoptive response of bone marrow cells to a thymus cell-independent antigen. Immunology. 1974 Nov;27(5):795–806. [PMC free article] [PubMed] [Google Scholar]
- Wargon L. B., Lozzio B. B., Wust C. J. Alteration of bone marrow-thymus cell synergism in hereditary asplenic and adult splenctomiced mice. Proc Soc Exp Biol Med. 1975 Mar;148(3):925–928. doi: 10.3181/00379727-148-38661. [DOI] [PubMed] [Google Scholar]
- Zierott G., Zenker M., Thiede A. Veränderungen der Serumimmunglobulinkonzentrationen bei splenektomierten Patienten. Bruns Beitr Klin Chir (1971) 1973 Dec;220(8):804–808. [PubMed] [Google Scholar]


