Abstract
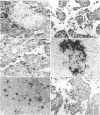
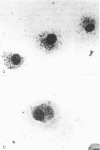
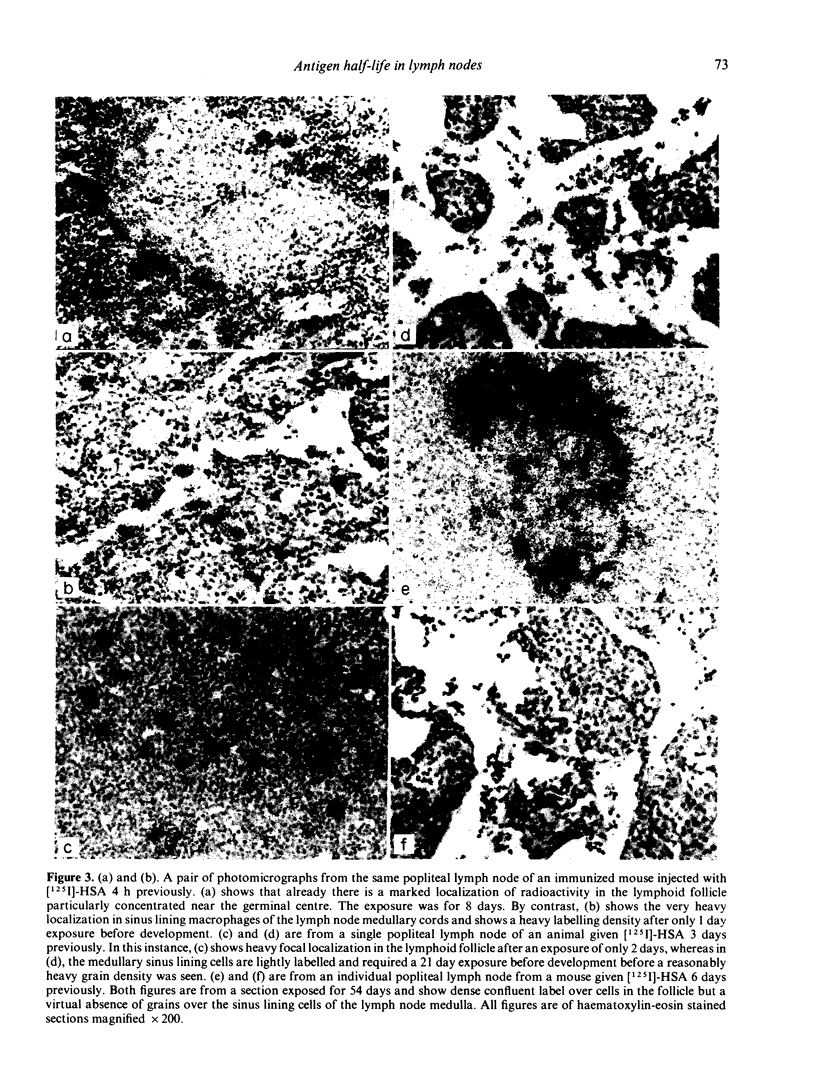
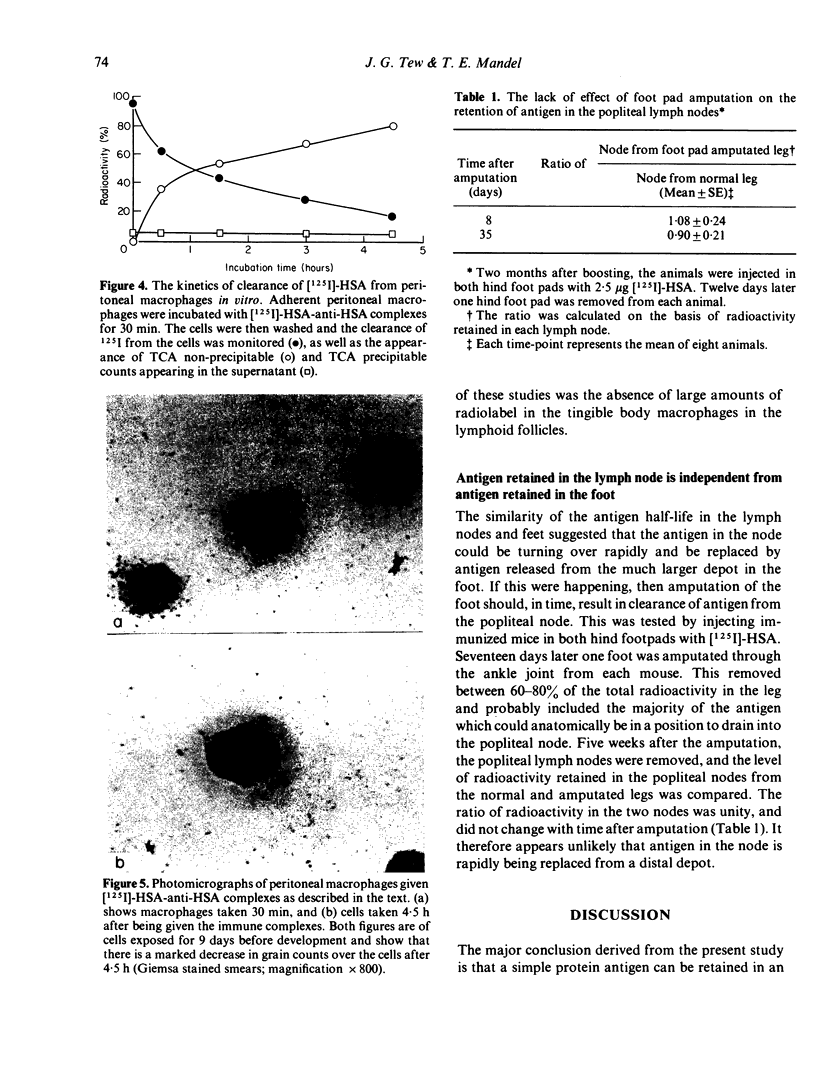
The kinetics of clearance of 125I from the popliteal lymph nodes and feet of human serum albumin (HSA)-immunized mice was studied following the injection of [125I]-HSA into the hind footpads. Antigen was cleared from both locations rapidly for the first few days. The antigen half-life (T½) during this period was only a matter of hours. By the end of the first week, however, the rate of clearance in both sites had changed markedly. The antigen T½ in the node between the first and sixth week was 8.1 weeks (95% confidence interval between 5.1 and 20 weeks) and the antigen T½ in the foot was 6.1 weeks (95% confidence interval between 3.7 and 16.6 weeks). There was, however, about twenty times more radioactivity in the feet than in the popliteal nodes. Autoradiography of popliteal lymph nodes revealed that initially antigen was trapped in the medulla, subcapsular sinus, superficial cortex and around lymphoid follicles. During the first few days antigen was cleared from all sites except the follicles. The radioactivity initially trapped in the medulla, subcapsular sinus, and superficial cortex appeared to have been associated with macrophages. Studies with peritoneal macrophages indicated an antigen T½ in these cells of 2 h (95% confidence interval between 1.5 and 3 h). The initial rapid clearance of antigen trapped and catabolized by macrophages and the long-term retention of antigen in the follicles is probably attributable to trapping and retention by follicular dendritic cells. The large pool of antigen trapped in the foot did not appear to serve as a depot to replace antigen degraded in the node, since amputation of the foot did not alter the level of antigen retained in the node. The long antigen T½ in the lymph node follicles indicates that antigen is available in the lymph node to play a role in the maintenance and regulation of immune responses for many months or even years.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., NOSSAL G. J., PYE J. ANTIGENS IN IMMUNITY. III. DISTRIBUTION OF IODINATED ANTIGENS FOLLOWING INJECTION INTO RATS VIA THE HIND FOOTPADS. Aust J Exp Biol Med Sci. 1964 Jun;42:295–310. [PubMed] [Google Scholar]
- Britton S., Celada F. Immunogenicity of human serum albumin: decay in the normal mouse. Immunology. 1968 Apr;14(4):503–509. [PMC free article] [PubMed] [Google Scholar]
- Britton S., Möller G. Regulation of antibody synthesis against Escherichia coli endotoxin. I. Suppressive effect of endogenously produced and passively transferred antibodies. J Immunol. 1968 Jun;100(6):1326–1334. [PubMed] [Google Scholar]
- Bystryn J. C., Graf M. W., Uhr J. W. Regulation of antibody formation by serum antibody. II. Removal of specific antibody by means of exchange transfusion. J Exp Med. 1970 Dec 1;132(6):1279–1287. doi: 10.1084/jem.132.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf M. W., Uhr J. W. Regulation of antibody formation by serum antibody. I. Removal of specific antibody by means of immunoadsorption. J Exp Med. 1969 Nov 1;130(5):1175–1186. doi: 10.1084/jem.130.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E. J., Tew J. G., Stavitsky A. B. The differential localization of the in vitro spontaneous antibody and proliferative responses in lymphoid organs proximal and distal to the site of primary immunization. Cell Immunol. 1975 Aug;18(2):476–483. doi: 10.1016/0008-8749(75)90074-x. [DOI] [PubMed] [Google Scholar]
- Kitces E. N., Tew J. G., Green E. J. Induction and maintenance of the antibody response by different forms of human serum albumin. Immunol Commun. 1975;4(3):275–278. doi: 10.3109/08820137409055779. [DOI] [PubMed] [Google Scholar]
- Klaus G. G. The generation of memory cells. II. Generation of B memory cells with preformed antigen-antibody complexes. Immunology. 1978 Apr;34(4):643–652. [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. Unmasking of cell-associated foreign antigens during incubation of lymphoid cells. Isr J Med Sci. 1969 Mar-Apr;5(2):230–234. [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J., Lummus Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J Exp Med. 1968 Feb 1;127(2):277–290. doi: 10.1084/jem.127.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein I., Bystryn J. C., Uhr J. W. Sepcific removal of in vivo antibody by extracorporeal circulation over an immunoadsorbent in gel. J Clin Invest. 1971 Sep;50(9):1864–1868. doi: 10.1172/JCI106678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke J. R., Unanue E. R. Macrophage-antigen interaction: uptake, metabolism and immunogenicity of foreign albumin. J Immunol. 1971 Aug;107(2):331–338. [PubMed] [Google Scholar]
- Tew J. G., Greene E. J., Makoski M. H. In vitro evidence indicating a role for the Fc region of IgG in the mechanism for the long-term maintenance and regulation of antibody levels in vivo. Cell Immunol. 1976 Oct;26(2):141–152. doi: 10.1016/0008-8749(76)90358-0. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Mandel T. The maintenance and regulation of serum antibody levels: evidence indicating a role for antigen retained in lymphoid follicles. J Immunol. 1978 Mar;120(3):1063–1069. [PubMed] [Google Scholar]
- Tew J. G., Self C. H., Harold W. W., Stavitsky A. B. The spontaneous induction of anamnestic antibody synthesis in lymph node cell cultures many months after primary immunization. J Immunol. 1973 Aug;111(2):416–423. [PubMed] [Google Scholar]
- Weigle W. O. Cyclical production of antibody as a regulatory mechanism in the immune response. Adv Immunol. 1975;21:87–111. doi: 10.1016/s0065-2776(08)60219-9. [DOI] [PubMed] [Google Scholar]