Abstract
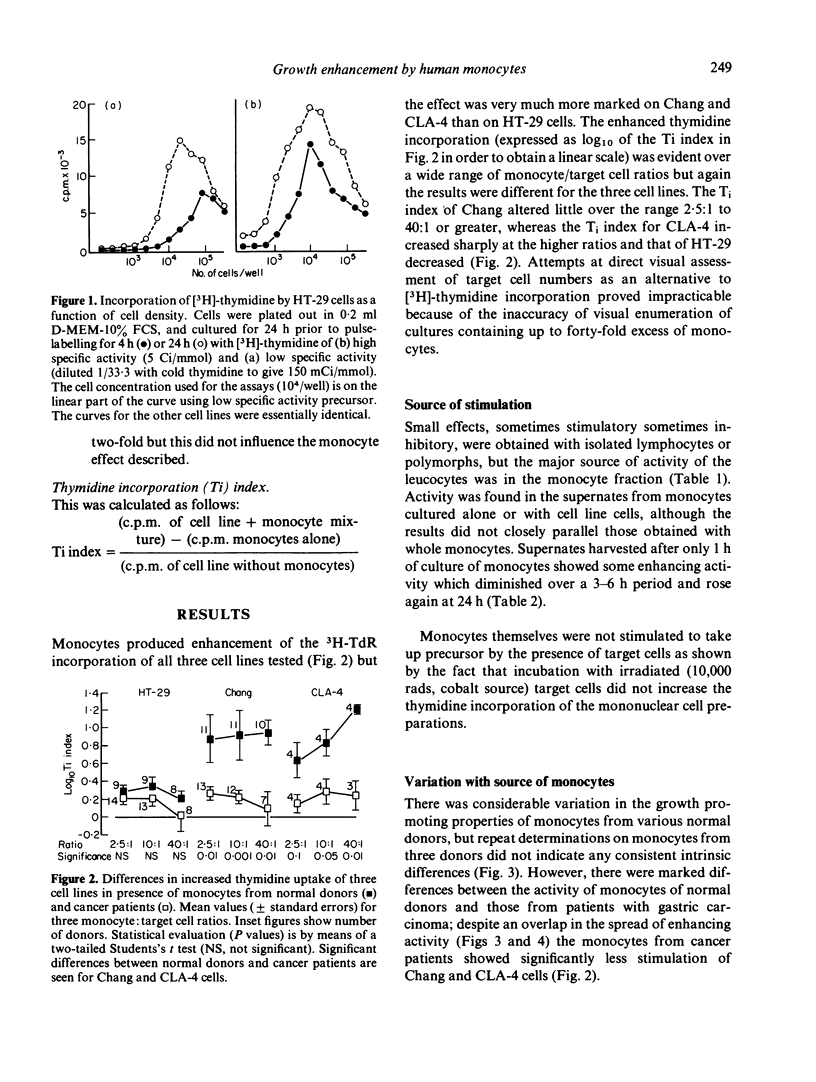
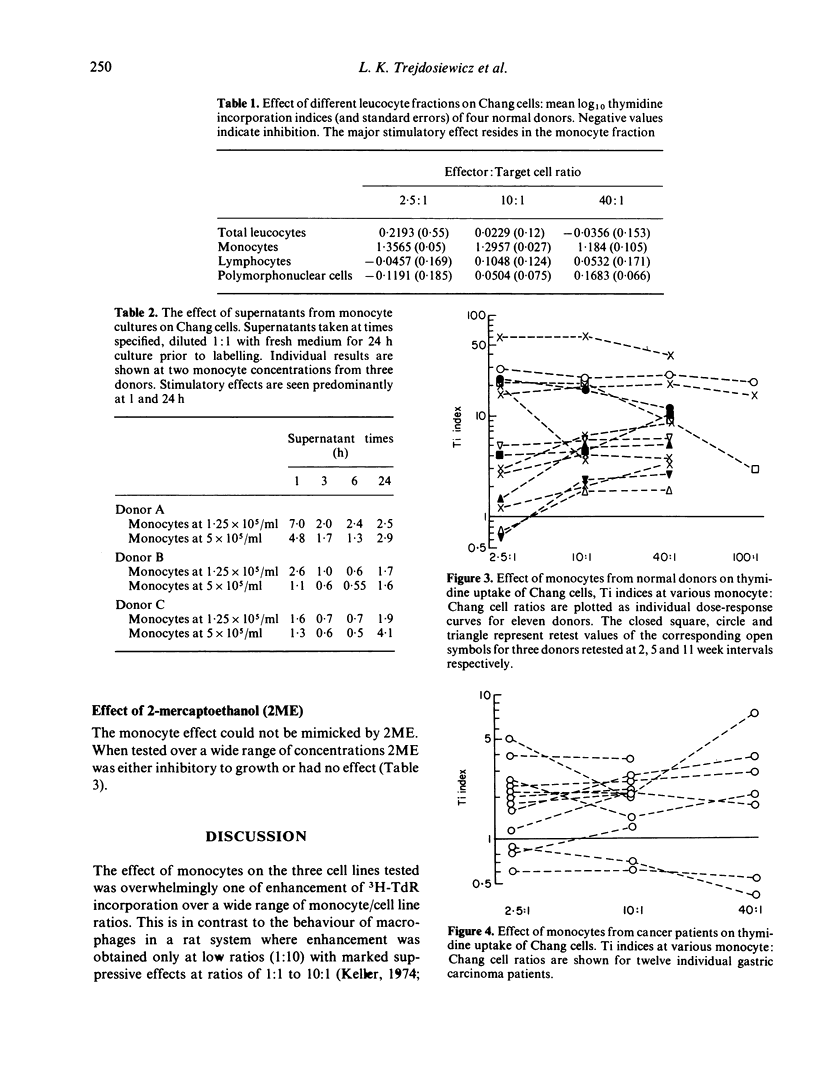
Peripheral blood monocytes, but not other leucocytes, from healthy donors, were shown to enhance the DNA synthesis of three cell lines. The effect was very marked on an epithelial (Chang) and lymphoid (CLA-4) cell line derived from normal tissues, and less marked on a carcinoma-derived line (HT-29). The enhancement was demonstrable over a wide range of monocyte:cell line ratios, and some activity was present in supernates from monocytes cultured alone or with a cell line. Furthermore, monocytes from gastric carcinoma patients did not enhance Chang and CLA-4 cells to the same extent, relative to the healthy donors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahern T., Taylor G. A., Sanderson C. J. An evaluation of an assay for DNA synthesis in lymphocytes with [3H]thymidine and harvesting on to glass fibre filter discs. J Immunol Methods. 1976;10(4):329–336. doi: 10.1016/0022-1759(76)90027-2. [DOI] [PubMed] [Google Scholar]
- Alexander P. Letter: Surveillance against neoplastic cells-is it mediated by macrophages? Br J Cancer. 1976 Mar;33(3):344–345. doi: 10.1038/bjc.1976.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG R. S. M. Continuous subcultivation of epithelial-like cells from normal human tissues. Proc Soc Exp Biol Med. 1954 Nov;87(2):440–443. doi: 10.3181/00379727-87-21406. [DOI] [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Click R. E. Immune responses in vitro. V. Role of mercaptoethanol in the mixed-leukocyte reaction. Cell Immunol. 1972 Nov;5(3):410–418. doi: 10.1016/0008-8749(72)90067-6. [DOI] [PubMed] [Google Scholar]
- Keller R. Modulation of cell proliferation by macrophages: a possible function apart from cytotoxic tumour rejection. Br J Cancer. 1974 Nov;30(5):401–415. doi: 10.1038/bjc.1974.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Terry W. D. Differential stimulation of murine lymphoma growth in vitro by normal and BCG-activated macrophages. J Exp Med. 1975 Oct 1;142(4):887–902. doi: 10.1084/jem.142.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury K. C. In vitro stimulation and inhibition of tumor cell growth mediated by different lymphoid cell populations. Cancer Res. 1977 May;37(5):1408–1415. [PubMed] [Google Scholar]
- Opitz H. G., Niethammer D., Lemke H., Flad H. D., Huget R. Inhibition of 3H-thymidine incorporation of lymphocytes by a soluble factor from macrophages. Cell Immunol. 1975 Apr;16(2):379–388. doi: 10.1016/0008-8749(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Altered expression of human monocyte Fc receptors in malignant disease. Nature. 1977 Jan 20;265(5591):253–255. doi: 10.1038/265253a0. [DOI] [PubMed] [Google Scholar]
- Steel C. M., Edmond E. Human lymphoblastoid cell lines. I. Culture methods and examination for Epstein-Barr virus. J Natl Cancer Inst. 1971 Dec;47(6):1193–1201. [PubMed] [Google Scholar]
- Unanue E. R., Kíely J. M. Synthesis and secretion of a mitogenic protein by macrophages: description of a superinduction phenomenon. J Immunol. 1977 Sep;119(3):925–931. [PubMed] [Google Scholar]


