Abstract
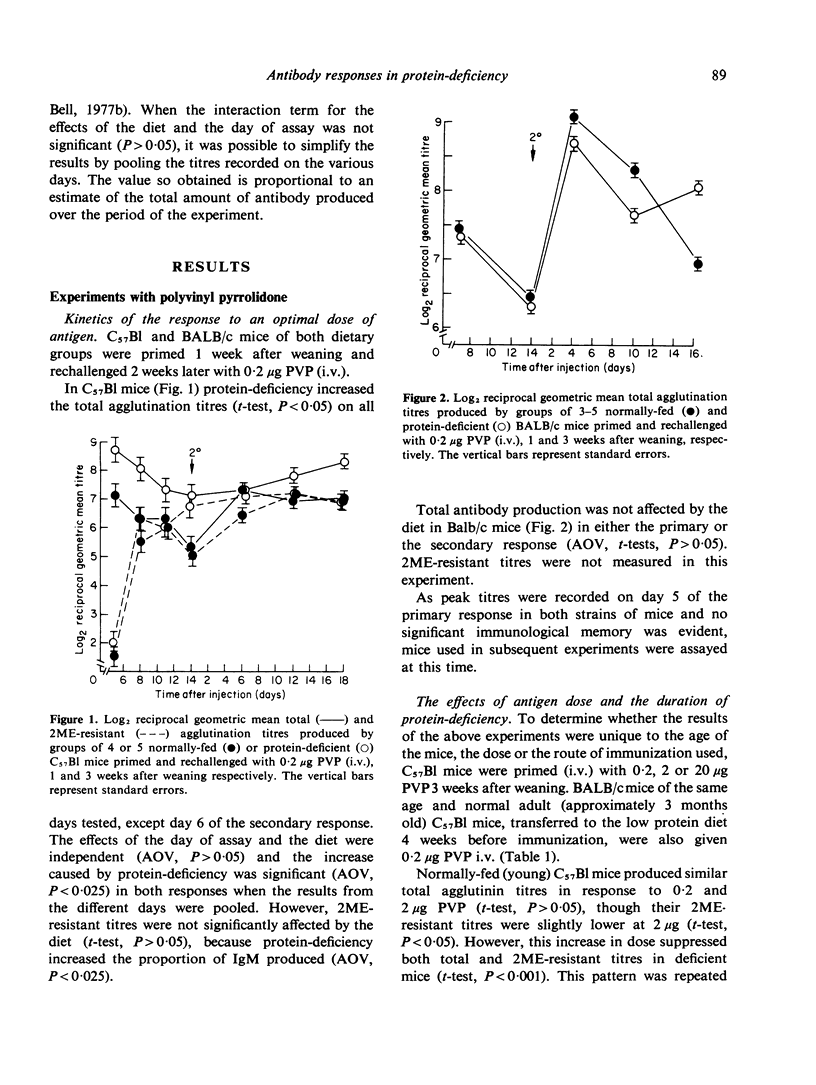
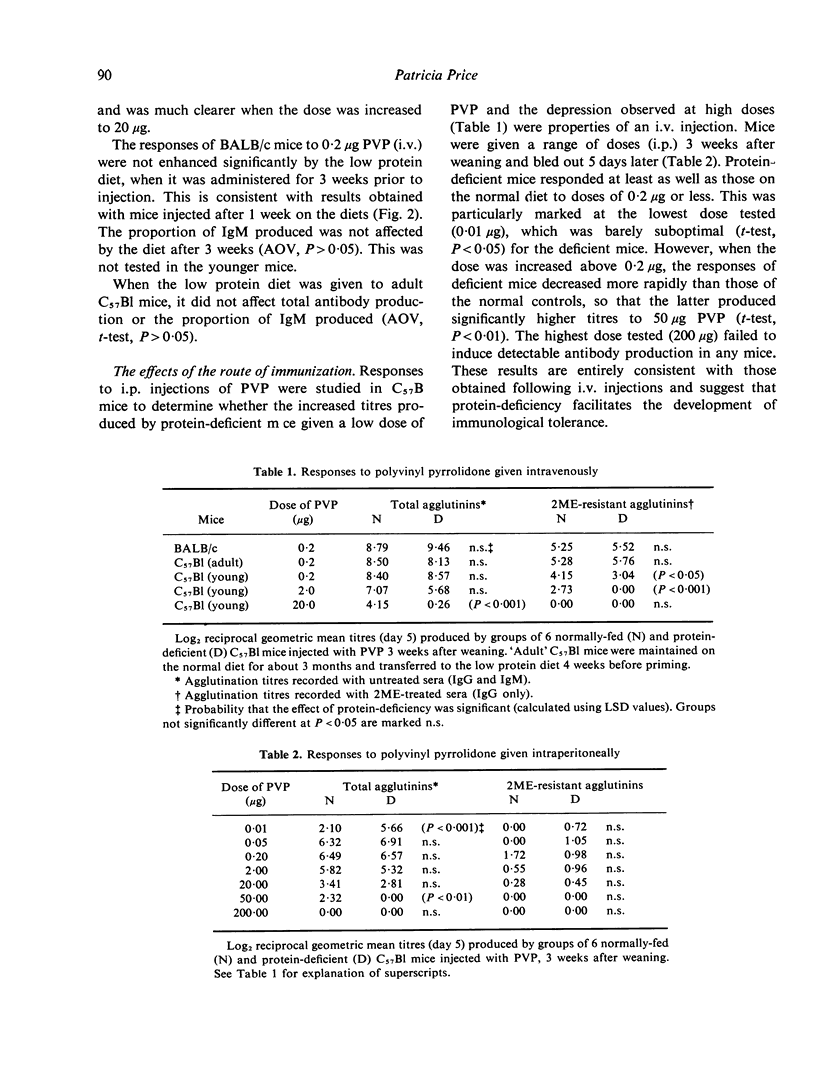
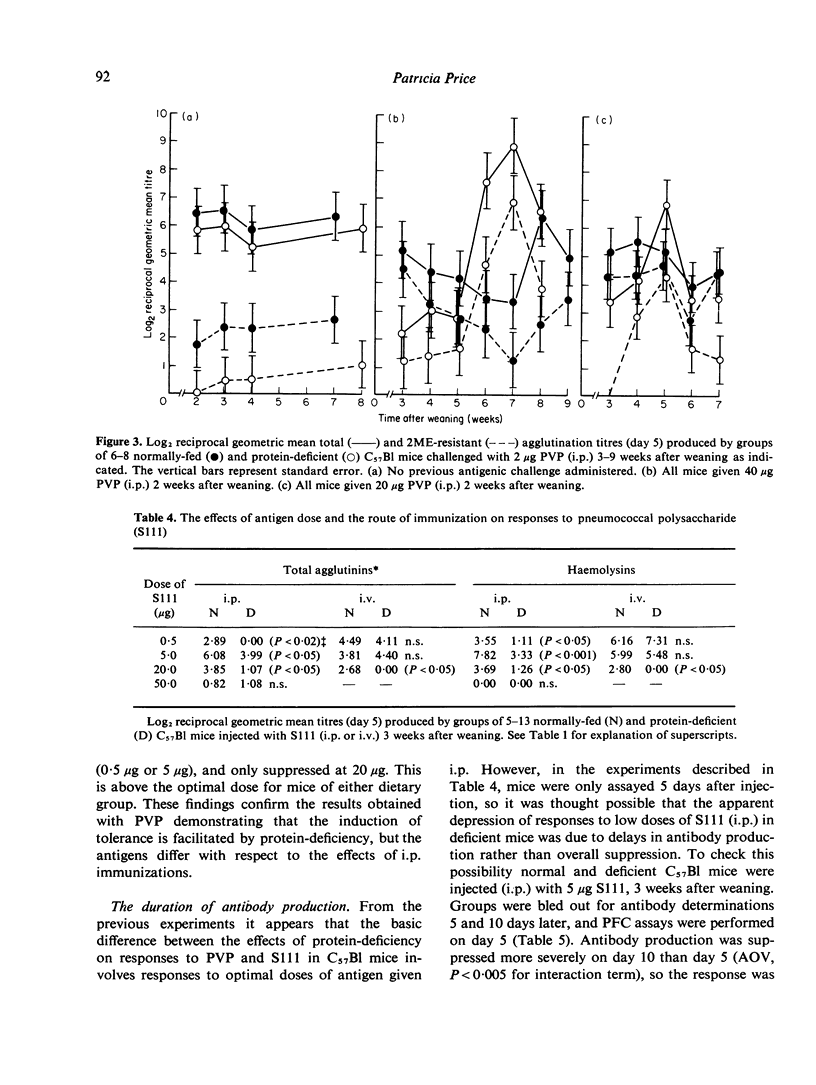
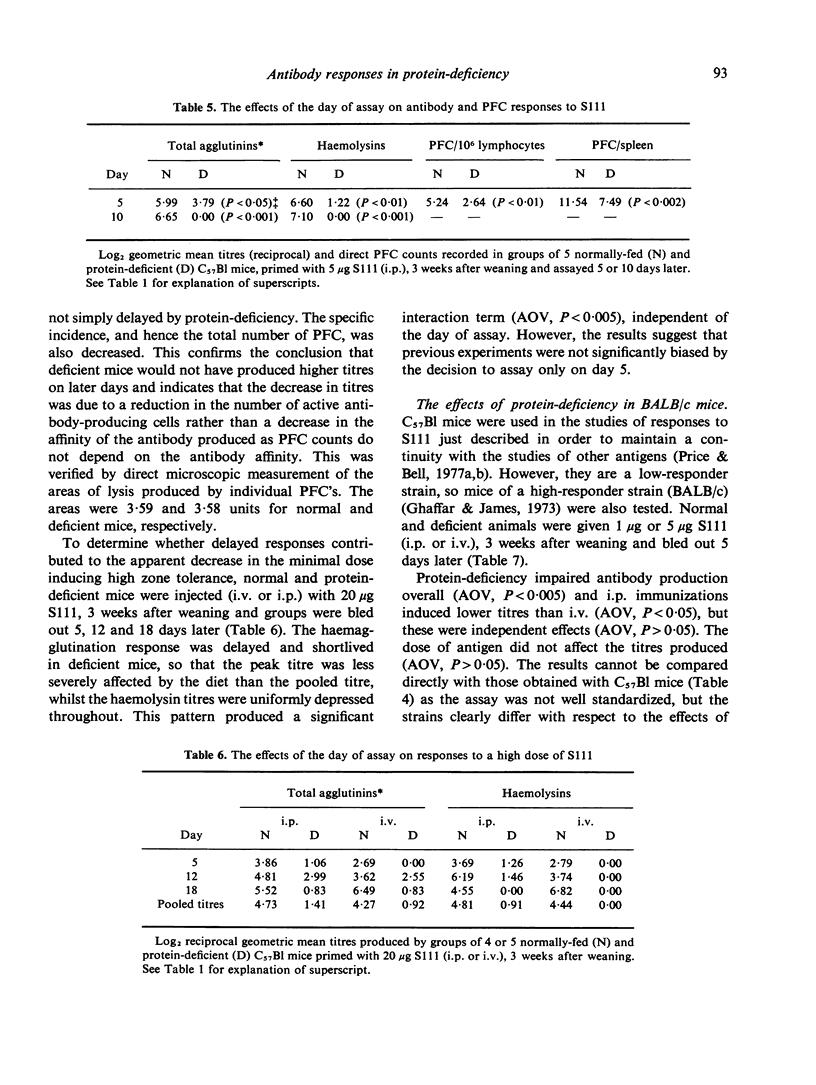
Indirect haemagglutination titres induced by polyvinyl pyrrolidone (PVP) or pneumococcal polysaccharide Type III (S111) were determined in mice maintained on a 4% albumin diet from weaning and normally-fed littermates. Responses to PVP, given intravenously (i.v.) or intraperitoneally (i.p.), were elevated by protein-deficiency at low antigen doses and increasingly depressed at high doses. Increases in the duration of protein-deficiency generally improved these responses. The persistence of tolerance was reduced by protein-deficiency and priming was evident in both groups when tolerance was broken. The low protein diet depressed responses to moderate doses of S111 given i.p. to C57Bl mice, but such responses were normal in BALB/c mice and in C57Bl mice injected i.v. High doses of S111 (i.p., i.v.) elicited poor responses in deficient mice. These findings are discussed in relation to previous studies using other antigens, with a view to elucidating mechanisms responsible for the effects of protein-deficiency.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., FARTHING C. P., HUMPHREY J. H. The significance of multiple antibody components in serum of immunized rabbits. Immunology. 1960 Oct;3:336–351. [PMC free article] [PubMed] [Google Scholar]
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Andersson B. Induction of immunity and immunologic paralysis in mice against polyvinyl pyrrolidone. J Immunol. 1969 May;102(5):1309–1313. [PubMed] [Google Scholar]
- Baker P. J., Prescott B. Letter: The basis for conflicting results obtained in studies on the plaque-forming cell response to type III pneumococcal polysaccharide. J Immunol. 1975 Sep;115(3):891–892. [PubMed] [Google Scholar]
- Bell R. G., Hazell L. A., Price P. Influence of dietary protein restriction on immune competence. II. Effect on lymphoid tissue. Clin Exp Immunol. 1976 Nov;26(2):314–326. [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H. Regulatory role of T cells in IgG antibody formation and immune memory to type III Pneumococcal polysaccharide. J Immunol. 1974 Dec;113(6):1909–1920. [PubMed] [Google Scholar]
- Chandra R. K. Immunocompetence in undernutrition. J Pediatr. 1972 Dec;81(6):1194–1200. doi: 10.1016/s0022-3476(72)80262-2. [DOI] [PubMed] [Google Scholar]
- Chaouat G., Howard J. G. Influence of reticuloendothelial blockade on the induction of tolerance and immunity by polysaccharides. Immunology. 1976 Feb;30(2):221–227. [PMC free article] [PubMed] [Google Scholar]
- Cooper W. C., Good R. A., Mariani T. Effects of protein insufficiency on immune responsiveness. Am J Clin Nutr. 1974 Jun;27(6):647–664. doi: 10.1093/ajcn/27.6.647. [DOI] [PubMed] [Google Scholar]
- Coovadia H. M., Soothill J. F. The effect of amino acid restricted diets on the clearance of 125I-labelled polyvinyl pyrrolidone in mice. Clin Exp Immunol. 1976 Mar;23(3):562–567. [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J. A method of increased sensitivity for detecting single antibody-forming cells. Nature. 1965 Sep 4;207(5001):1106–1107. doi: 10.1038/2071106a0. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Demaeyer E. M., Davies A. J. Some effects of malnutrition on the immune response in man. Am J Clin Nutr. 1974 Jun;27(6):638–646. doi: 10.1093/ajcn/27.6.638. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Influence of protein restriction on immune functions in NZB mice. J Immunol. 1976 Mar;116(3):782–790. [PubMed] [Google Scholar]
- Fujiwara M. Activation and suppression of T cells in termination of immunological tolerance. Immunology. 1976 Nov;31(5):807–812. [PMC free article] [PubMed] [Google Scholar]
- Ghaffar A., James K. The effect of antilymphocytic antibody on the humoral immune response in different strains of mice. 3. The response to type 3 pneumococcus polysaccharide. Immunology. 1973 Jun;24(6):1075–1085. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M. Studies on immunological paralysis. IV. The relative contributions of continuous antibody neutralization and central inhibition to paralysis with type 3 pneumococcal polysaccharide. Proc R Soc Lond B Biol Sci. 1971 Sep 28;178(1053):417–438. doi: 10.1098/rspb.1971.0073. [DOI] [PubMed] [Google Scholar]
- James K., Milne I. The effect of antilymphocytic antibody on the primary immune response of mice to sheep erythrocytes, bovine serum albumin, and type 3 pneumococcus polysaccharide. Transplantation. 1971 Aug;12(2):109–113. doi: 10.1097/00007890-197108000-00002. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Enhanced immune responsiveness to a thymus-independent antigen early after adult thymectomy: evidence for short-lived inhibitory thymus-derived cells. Eur J Immunol. 1972 Apr;2(2):114–118. doi: 10.1002/eji.1830020204. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Variable effects of anti-lymphocyte serum on humoral antibody formation: role of thymus dependency of antigen. J Immunol. 1971 Apr;106(4):917–926. [PubMed] [Google Scholar]
- Mitchell G. F. T cell modification of B cell responses to antigen in mice. Contemp Top Immunobiol. 1974;3:97–116. doi: 10.1007/978-1-4684-3045-5_4. [DOI] [PubMed] [Google Scholar]
- Polak L., Turk J. L. Reversal of immunological tolerance by cyclophosphamide through inhibition of suppressor cell activity. Nature. 1974 Jun 14;249(458):654–656. doi: 10.1038/249654a0. [DOI] [PubMed] [Google Scholar]
- Price P., Bell R. G. The toxicity of inactivated bacteria and endotoxin in mice suffering from protein malnutrition. J Reticuloendothel Soc. 1975 Oct;18(4):230–243. [PubMed] [Google Scholar]
- Rotter V., Trainin N. Thymus cell population exerting a regulatory function in the immune response of mice to polyvinyl pyrrolidone. Cell Immunol. 1974 Jul;13(1):76–86. doi: 10.1016/0008-8749(74)90228-7. [DOI] [PubMed] [Google Scholar]
- Sljivić V. S., Warr G. W. Activity of the reticuloendothelial system and the antibody response. III. The fate of type III pneumococcal polysaccharide and the antibody response. Immunology. 1974 Dec;27(6):1009–1022. [PMC free article] [PubMed] [Google Scholar]
- Stark J. M. Rate of antigen catabolism and immunogenicity of [131-I]BGG in mice. II. Immunogenicity of [131-I]BGG and adjuvant action after alteration of the metabolic rate by various means. Immunology. 1970 Sep;19(3):457–464. [PMC free article] [PubMed] [Google Scholar]
- Sterzl J. Immunological tolerance as the result of terminal differentiation of immunologically competent cells. Nature. 1966 Jan 22;209(5021):416–417. doi: 10.1038/209416a0. [DOI] [PubMed] [Google Scholar]
- Warr G. W., Ghaffar A., James K. The response of mice to type III pneumococcal polysaccharide: failure to detect thymus-derived suppressor cells. Cell Immunol. 1975 Jun;17(2):366–373. doi: 10.1016/s0008-8749(75)80040-2. [DOI] [PubMed] [Google Scholar]


