Abstract
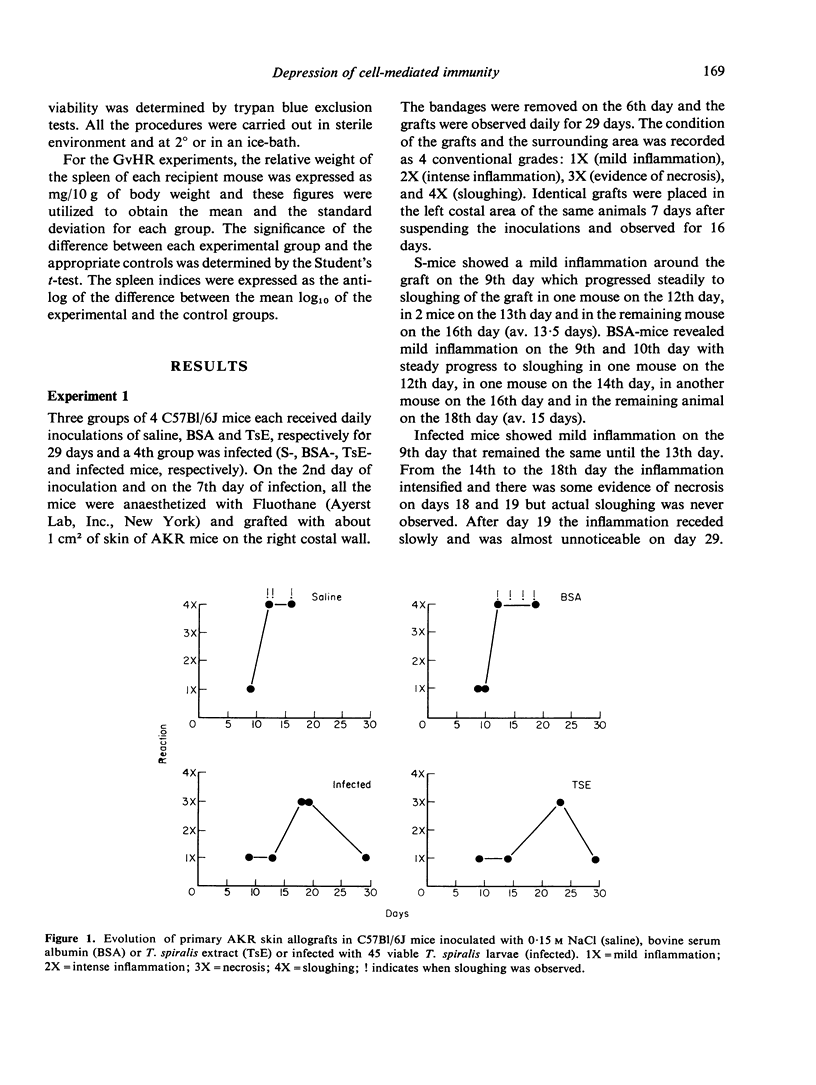
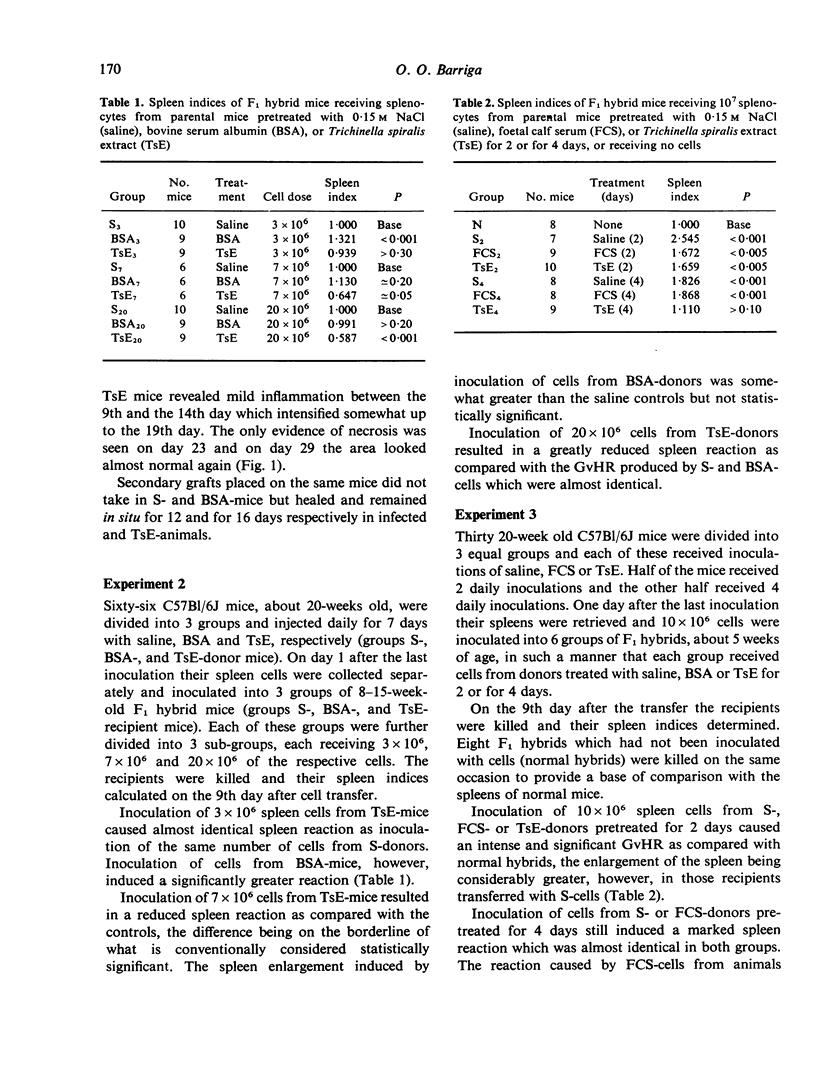
Mice pretreated with Trichinella spiralis extract (TsE), or infected with the parasite, rejected primary skin allografts in 18-23 days and secondary allografts in 12-16 days. Mice pretreated with saline or with bovine serum albumin (BSA) rejected the primary allografts in 12-18 days and did not accept the secondary grafts. Inoculation of increasing doses of parental spleen cells from mice pretreated with saline or with BSA in F1 hybrids produced proportionately stronger graft-versus-host reactions (GvHR) whereas increasing doses of cells from TsE pretreated mice reduced proportionately the capacity of the inoculum to induce a GvHR. Immunodepression of the parental cells was obtained with 7 and with 4, but not with 2, daily injections of TsE. The depression waned rapidly after the treatment with TsE but a significant degree still remained after 3 days. Immunodepression by TsE cannot be solely explained by antigenic competition and although our results are consistent with the induction of suppressor cells, it is probable that other mechanisms are also involved.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose C. T. Regulation of the secondary antibody response in vitro. Enhancement by actinomycin D and inhibition by a macromolecular product of stimulated lymph node cultures. J Exp Med. 1969 Nov 1;130(5):1003–1029. doi: 10.1084/jem.130.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. II. Mode of action of thymic-derived suppressor cells. J Immunol. 1974 Jan;112(1):404–409. [PubMed] [Google Scholar]
- Barriga O. O. Selective immunodepression in mice by Trichinella spiralis extracts and infections. Cell Immunol. 1975 May;17(1):306–309. doi: 10.1016/s0008-8749(75)80031-1. [DOI] [PubMed] [Google Scholar]
- Burns F. D., Marrack P. C., Kappler J. W., Janeway C. A., Jr Functional heterogeneity among the T-derived lymphocytes of the mouse. IV. Nature of spontaneously induced suppressor cells. J Immunol. 1975 Apr;114(4):1345–1347. [PubMed] [Google Scholar]
- Faubert G. M. Depression of the plaque-forming cells to sheep red blood cells by the new-born larvae of Trichinella spiralis. Immunology. 1976 Apr;30(4):485–489. [PMC free article] [PubMed] [Google Scholar]
- Faubert G. M., Tanner C. E. Leucoagglutination and cytotoxicity of the serum of infected mice and of extracts of Trichinella spiralis larvae and the capacity of infected mouse sera to prolong skin allografts. Immunology. 1975 Jun;28(6):1041–1050. [PMC free article] [PubMed] [Google Scholar]
- Faubert G. M., Tanner C. E. The suppression of sheep rosette-forming cells and the inability of mouse bone marrow cells to reconstitute competence after infbction with the nematode Trichinella spiralis. Immunology. 1974 Sep;27(3):501–505. [PMC free article] [PubMed] [Google Scholar]
- Faubert G., Tanner C. E. Trichinella spiralis: inhibition of sheep hemagglutinins in mice. Exp Parasitol. 1971 Aug;30(1):120–123. doi: 10.1016/0014-4894(71)90077-4. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Kontiainen S. Suppressor cell induction in vitro. II. Cellular requirements of suppressor cell induction. Eur J Immunol. 1976 Apr;6(4):302–305. doi: 10.1002/eji.1830060413. [DOI] [PubMed] [Google Scholar]
- Ford W. L., Simmonds S. J., Atkins R. C. Early cellular events in a systemic graft-vs.-host reaction. II. Autoradiographic estimates of the frequency of donor lymphocytes which respond to each Ag-B-determined antigenic complex. J Exp Med. 1975 Mar 1;141(3):681–696. doi: 10.1084/jem.141.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe S. C., Streilein J. W. Graft-versus-Host reactions: a review. Adv Immunol. 1976;22:119–221. doi: 10.1016/s0065-2776(08)60549-0. [DOI] [PubMed] [Google Scholar]
- Harley J. P., Gallicchio V. Trichinella spiralis: migration of larvae in the rat. Exp Parasitol. 1971 Aug;30(1):11–21. doi: 10.1016/0014-4894(71)90064-6. [DOI] [PubMed] [Google Scholar]
- Lubiniecki A. S., Cypess R. H., Lucas J. P. Immune response to and distribution of sheep erythrocytes in Trichinella spiralis infected mice. Tropenmed Parasitol. 1974 Sep;25(3):345–349. [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. I. Regulation of mixed lymphocyte reactions by alloantigen-activated thymus-derived lymphocytes. J Exp Med. 1974 Dec 1;140(6):1588–1603. doi: 10.1084/jem.140.6.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg E. J., Duyzings M. J. An immuno-histological study of the immunological response of the rat to infection with Trichinella spiralis. J Comp Pathol. 1972 Oct;82(4):401–407. doi: 10.1016/0021-9975(72)90039-4. [DOI] [PubMed] [Google Scholar]
- Thomas D. W., Roberts W. K., Talmage D. W. Regulation of the immune response: production of a soluble suppressor by immune spleen cells in vitro. J Immunol. 1975 May;114(5):1616–1622. [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]


