Abstract
Congenitally athymic (nu/nu) mice and their thymus-bearing (+/nu) littermates were used to study the effect of a tapeworm, Hymenolepis diminuta, infection, particularly the worm kinetics and histopathology of the small intestine. Groups of nu/nu and +/nu mice were infected once with 5 cysticercoids and examined for 20 days post infection.
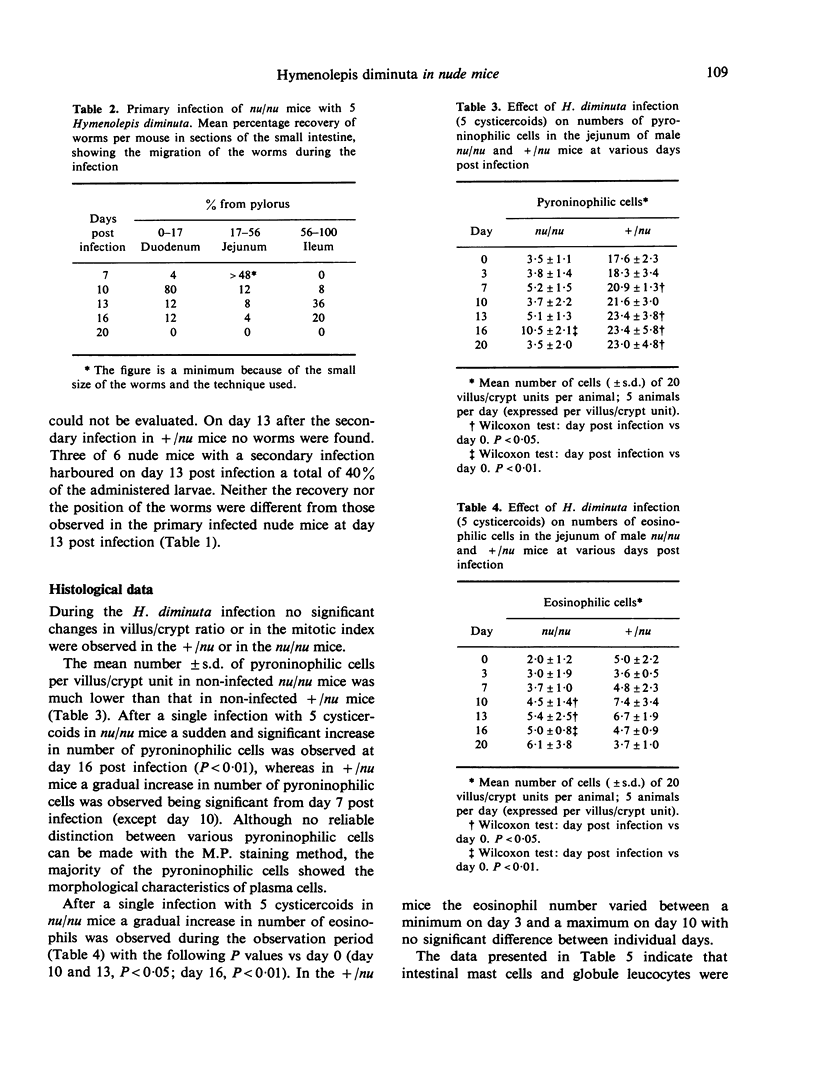
Worms were expelled both in nu/nu and in +/nu mice, albeit earlier in the latter animals. In both groups specific antibodies could be detected. The antibody titre was highest in the +/nu mice, which also formed more pyroninophilic, including plasma cells. The number of eosinophils increased significantly in the infected nu/nu mice, but not in the +/nu mice. A significant increase in mast cells and globule leucocyte formation was observed in the infected +/nu mice, but none of these cells were found in nu/nu mice which also expelled the worms. No changes in the villus/crypt ration in the jejunum were observed. The mitotic index of the epithelial crypt cells in the jejunum increased in the infected nu/nu mice reaching a peak at day 16 post infection.
After re-infection nu/nu mice were not able to expel worms earlier than after primary infection. In passive immunization experiments with serum from both infected nu/nu and +/nu mice no conclusive evidence was obtained for a role of serum antibodies in host protection. It was concluded that host protection to the tapeworm, H. diminuta was dependent on the number of worms and worms could be expelled in the absence of functional T-cells. The hypothesis was put forward that the functional antigens are related to the scolex region and not to the total worm mass.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basten A., Beeson P. B. Mechanism of eosinophilia. II. Role of the lymphocyte. J Exp Med. 1970 Jun 1;131(6):1288–1305. doi: 10.1084/jem.131.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. S. Studies on host resistance to secondary infections of Raillietina cesticillus Molin, 1858 in the fowl. Parasitology. 1973 Dec;67(3):375–382. doi: 10.1017/s003118200004659x. [DOI] [PubMed] [Google Scholar]
- Gray J. S. The cellular response of the fowl small intestine to primary and secondary infections of the cestode Raillietina cesticillus (Molin). Parasitology. 1976 Oct;73(2):189–204. doi: 10.1017/s0031182000046862. [DOI] [PubMed] [Google Scholar]
- Harris W. G., Turton J. A. Antibody response to tapeworm (Hymenolepis diminuta) in the rat. Nature. 1973 Dec 21;246(5434):521–522. doi: 10.1038/246521a0. [DOI] [PubMed] [Google Scholar]
- Hesselberg C. A., Andreassen J. Some influences of population density on Hymenolepis diminuta in rats. Parasitology. 1975 Dec;71(3):517–523. doi: 10.1017/s0031182000047272. [DOI] [PubMed] [Google Scholar]
- Hopkins C. A., Subramanian G., Stallard H. The development of Hymenolepis diminuta in primary and secondary infections in mice. Parasitology. 1972 Jun;64(3):401–412. doi: 10.1017/s0031182000045479. [DOI] [PubMed] [Google Scholar]
- Hopkins C. A., Subramanian G., Stallard H. The effect of immunosuppressants on the development of Hymenolepis diminuta in mice. Parasitology. 1972 Aug;65(1):111–120. doi: 10.1017/s0031182000044279. [DOI] [PubMed] [Google Scholar]
- Isaak D. D., Jacobson R. H., Reed N. D. Thymus dependence of tapeworm (Hymenolepis diminuta) elimination from mice. Infect Immun. 1975 Dec;12(6):1478–1479. doi: 10.1128/iai.12.6.1478-1479.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson R. H., Reed N. D. The immune response of congenitally athymic (nude) mice to the intestinal nematode Nippostrongylus brasiliensis. Proc Soc Exp Biol Med. 1974 Dec;147(3):667–670. doi: 10.3181/00379727-147-38412. [DOI] [PubMed] [Google Scholar]
- Jacobson R. H., Reed N. D. The thymus dependency of resistance to pinworm infection in mice. J Parasitol. 1974 Dec;60(6):976–979. [PubMed] [Google Scholar]
- Larsh J. E., Jr Allergic inflammation as a hypothesis for the expulsion of worms from tissues: a review. Exp Parasitol. 1975 Apr;37(2):251–266. doi: 10.1016/0014-4894(75)90077-6. [DOI] [PubMed] [Google Scholar]
- Miller H. R. Immune reactions in mucous membranes. II. The differentiation of intestinal mast cells during helminth expulsion in the rat. Lab Invest. 1971 May;24(5):339–347. [PubMed] [Google Scholar]
- Ogilvie B. M., Jones V. E. Parasitological review. Nippostrongylus brasiliensis: a review of immunity and host-parasite relationship in the rat. Exp Parasitol. 1971 Feb;29(1):138–177. doi: 10.1016/0014-4894(71)90021-x. [DOI] [PubMed] [Google Scholar]
- REILLY R. W., KIRSNER J. B. RUNT INTESTINAL DISEASE. Lab Invest. 1965 Jan;14:102–107. [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976 Nov 18;264(5583):258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A., Kruizinga W., Leenstra F. Trichinella spiralis infection in congenitally athymic (nude) mice. Parasitological, serological and haematological studies with observations on intestinal pathology. Immunology. 1977 Oct;33(4):581–587. [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg E. J., Steerenberg P. A. Intestinal phase of Trichinella spiralis in congenitally athymic (nude) mice. J Parasitol. 1974 Dec;60(6):1056–1057. [PubMed] [Google Scholar]
- Ruitenberg E. J., van der Sleen G. A modified immunofluorescence test for echinococcosis. J Parasitol. 1972 Apr;58(2):347–347. [PubMed] [Google Scholar]
- Turton J. A., Williamson J. R., Harris W. G. Haematological and immunological responses to the tapeworm hymenolepis diminuta in man. Tropenmed Parasitol. 1975 Jun;26(2):196–200. [PubMed] [Google Scholar]


