Abstract
The growth of a milk-sensitive strain of Escherichia coli in 1% peptone water can be inhibited for at least 3 h by IgA isolated from human milk or IgG1 from bovine colostrum acting with native iron-binding proteins from milk or serum. The immunoglobulins alone are inactive; the native iron-binding proteins alone are sometimes partially active. All this activity is inconsistent and not always enhanced by the addition of bicarbonate ions.
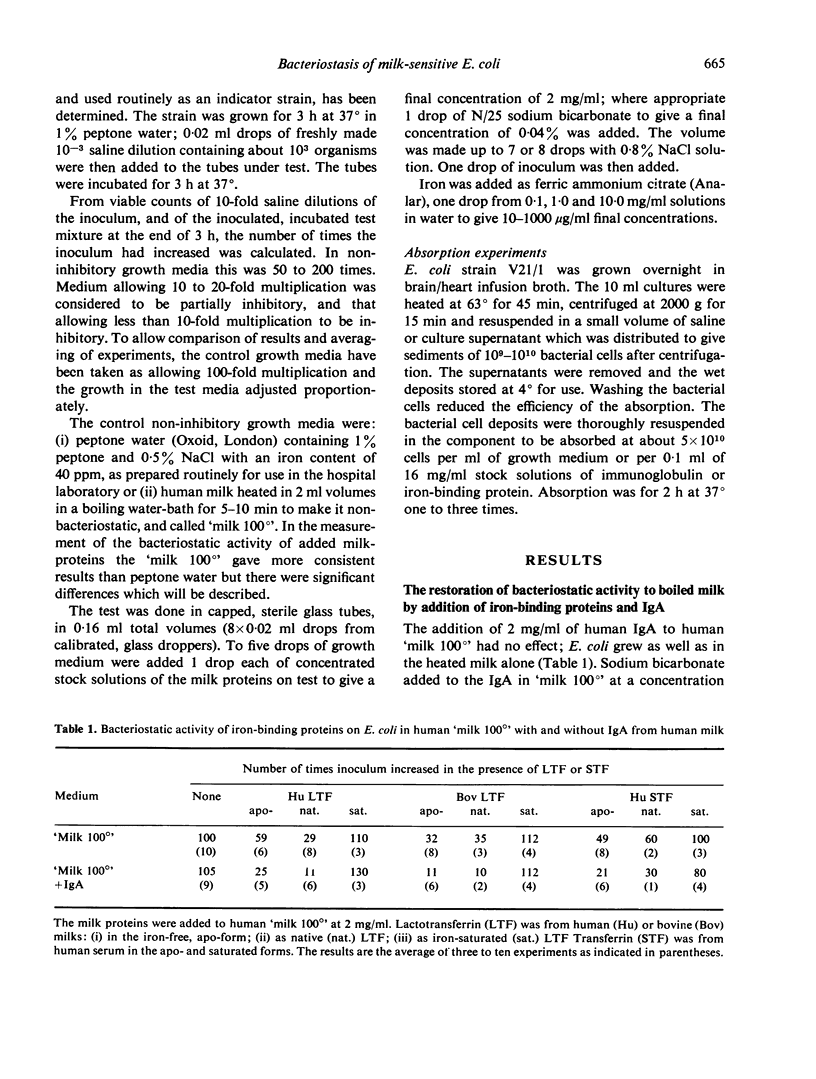
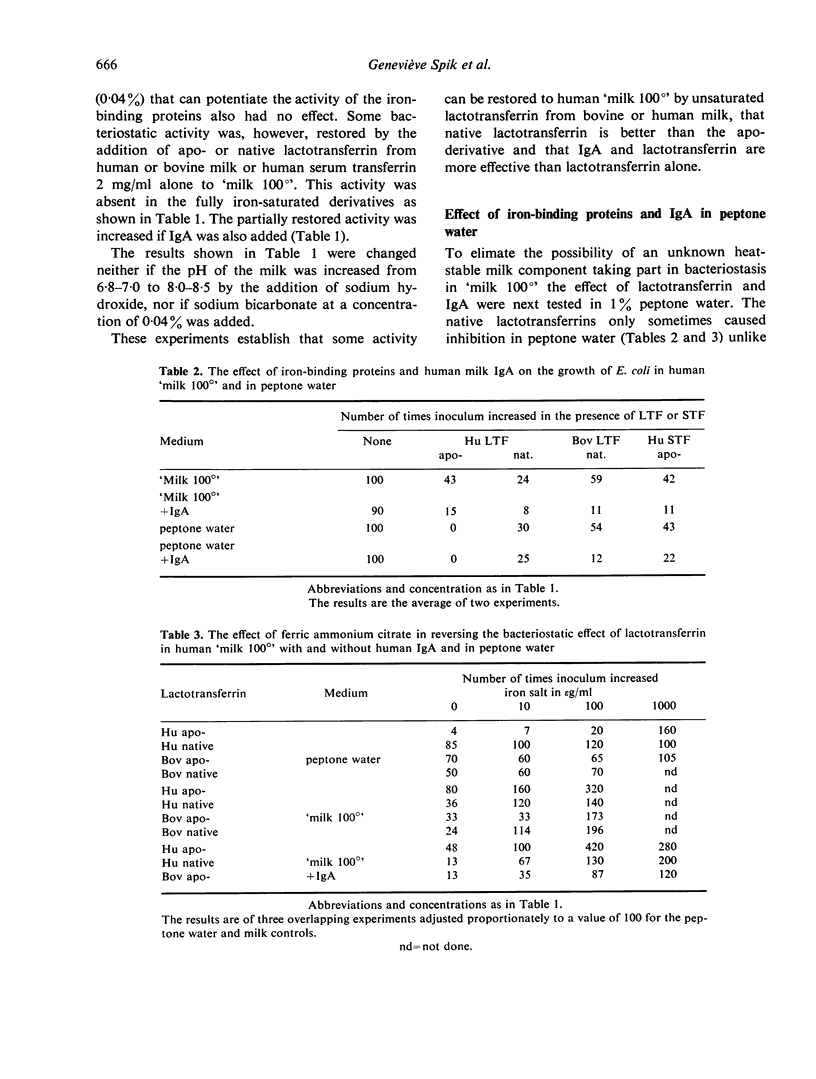
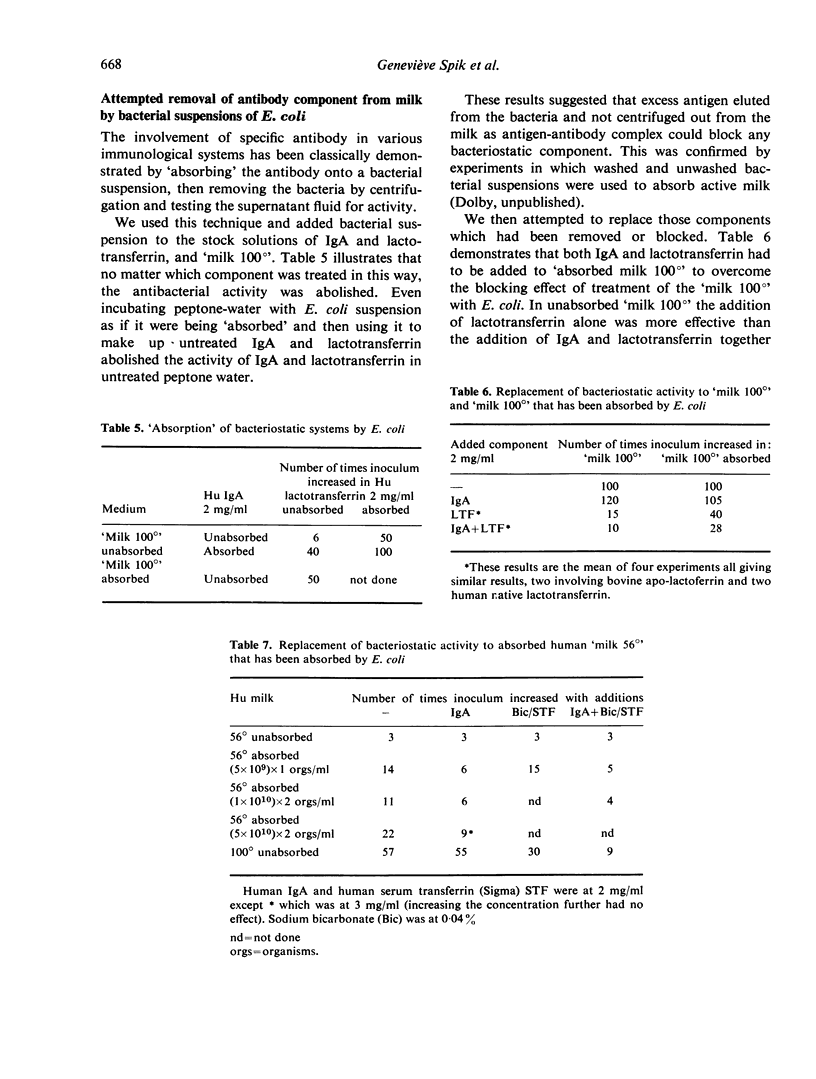
The growth of E. coli in human milk that has been inactivated by heating at 100° is consistently inhibited by IgA or IgG1 acting with native iron-binding proteins. The immunoglobulins are inactive alone but the iron-binding proteins have considerably more activity when added alone to inactivated milk than to peptone water, suggesting that the growth medium is contributing to or stabilising the activity. The addition of bicarbonate ions is without effect. Attempted absorption of antibody with suspensions of E. coli and replacement of bacteriostatic activity by addition of purified milk proteins has not, however, suggested any participants in the bacteriostasis of milk-sensitive strains other than antibody and iron-binding protein.
Bacteriostasis is abolished by saturating the transferrins with iron. The iron-free apo-derivatives are not more inhibitory than the native proteins except for human apo-lactotransferrin in peptone water which inhibits growth completely. This latter inhibition is not attributable to the low pH and 10–100 times more iron is needed to abolish this activity than is needed to abolish that of bovine apo-lactotransferrin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Aasa R., Malmström B. G., Vänngård T. Bicarbonate and the binding of iron to transferrin. J Biol Chem. 1967 May 25;242(10):2484–2490. [PubMed] [Google Scholar]
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal Biochem. 1971 Apr;40(2):450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Chéron A., Fournet B., Spik G., Montreuil J. Structure complète de glycopeptides isolés des immunoglobulines IgG1 du colostrum de vache. Biochimie. 1976;58(8):927–942. doi: 10.1016/s0300-9084(76)80281-7. [DOI] [PubMed] [Google Scholar]
- Chéron A., Mazurier J., Fournet B. Fractionnement chromatographique et études sur la microhétérogénété de la lactotransferrine de vache préparée par un procédé original. C R Acad Sci Hebd Seances Acad Sci D. 1977 Feb 14;284(7):585–588. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dolby J. M., Stephens S., Honour P. Bacteriostasis of Escherichia coli by milk. II. Effect of bicarbonate and transferrin on the activity of infant feeds. J Hyg (Lond) 1977 Apr;78(2):235–242. doi: 10.1017/s0022172400056126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone G. P., Walton E. Effect of iron on the bactericidal proteins from rabbit polymorphonuclear leukocytes. Nature. 1970 Aug 22;227(5260):849–851. doi: 10.1038/227849a0. [DOI] [PubMed] [Google Scholar]
- Goldblum R. M., Ahlstedt S., Carlsson B., Hanson L. A., Jodal U., Lidin-Janson G., Sohl-Akerlund A. Antibody-forming cells in human colostrum after oral immunisation. Nature. 1975 Oct 30;257(5529):797–798. doi: 10.1038/257797a0. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Humphreys J. Bacteriostatic effect of human milk and bovine colostrum on Escherichia coli: importance of bicarbonate. Infect Immun. 1977 Feb;15(2):396–401. doi: 10.1128/iai.15.2.396-401.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTREUIL J., CHOSSON A., HAVEZ R., MULLET S. [Isolation of beta2A-globulins from human milk]. C R Seances Soc Biol Fil. 1960;154:732–736. [PubMed] [Google Scholar]
- MONTREUIL J., MULLET S. [Isolation of lactosiderophilin from human milk]. C R Hebd Seances Acad Sci. 1960 Feb 29;250:1736–1737. [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Metal-combining properties of human lactoferrin (red milk protein). 1. The involvement of bicarbonate in the reaction. Eur J Biochem. 1968 Dec 5;6(4):579–584. doi: 10.1111/j.1432-1033.1968.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim Biophys Acta. 1968 Dec 23;170(2):351–365. doi: 10.1016/0304-4165(68)90015-9. [DOI] [PubMed] [Google Scholar]
- Raptopoulou-Gigi M., Marwick K., McClelland D. B. Antimicrobial proteins in sterilised human milk. Br Med J. 1977 Jan 1;1(6052):12–14. doi: 10.1136/bmj.1.6052.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reite B., Oram J. D. Bacterial inhibitors in milk and other biological fluids. Nature. 1967 Oct 28;216(5113):328–330. doi: 10.1038/216328a0. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Ferric iron and the antibacterial effects of horse 7S antibodies to Escherichia coli O111. Immunology. 1976 Mar;30(3):425–433. [PMC free article] [PubMed] [Google Scholar]
- Spik G. Formes conjuguées et assimilation du lactofer par le nourrisson. Etude particulière des lactotransferrines. Ann Nutr Aliment. 1971;25(2):A81–134. [PubMed] [Google Scholar]
- Spik G., Montreuil J. Etudes sur les glycoprotéins. 38. Démonstration de la nature N-(beta-aspartyl)-N-acetylglucosaminylamine de la liaison glucides-protéine dans la transferrine humaine. Bull Soc Chim Biol (Paris) 1969 Dec 18;51(9):1271–1285. [PubMed] [Google Scholar]
- Vaitukaitis J., Robbins J. B., Nieschlag E., Ross G. T. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971 Dec;33(6):988–991. doi: 10.1210/jcem-33-6-988. [DOI] [PubMed] [Google Scholar]


