Abstract
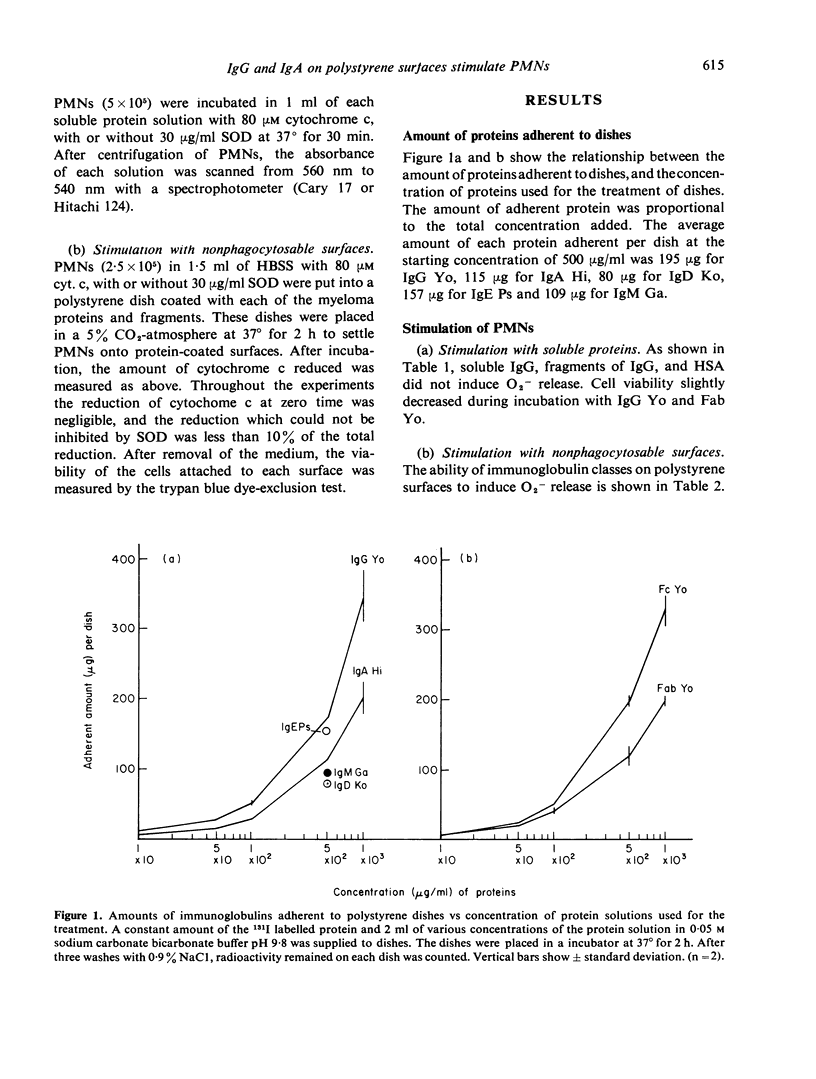
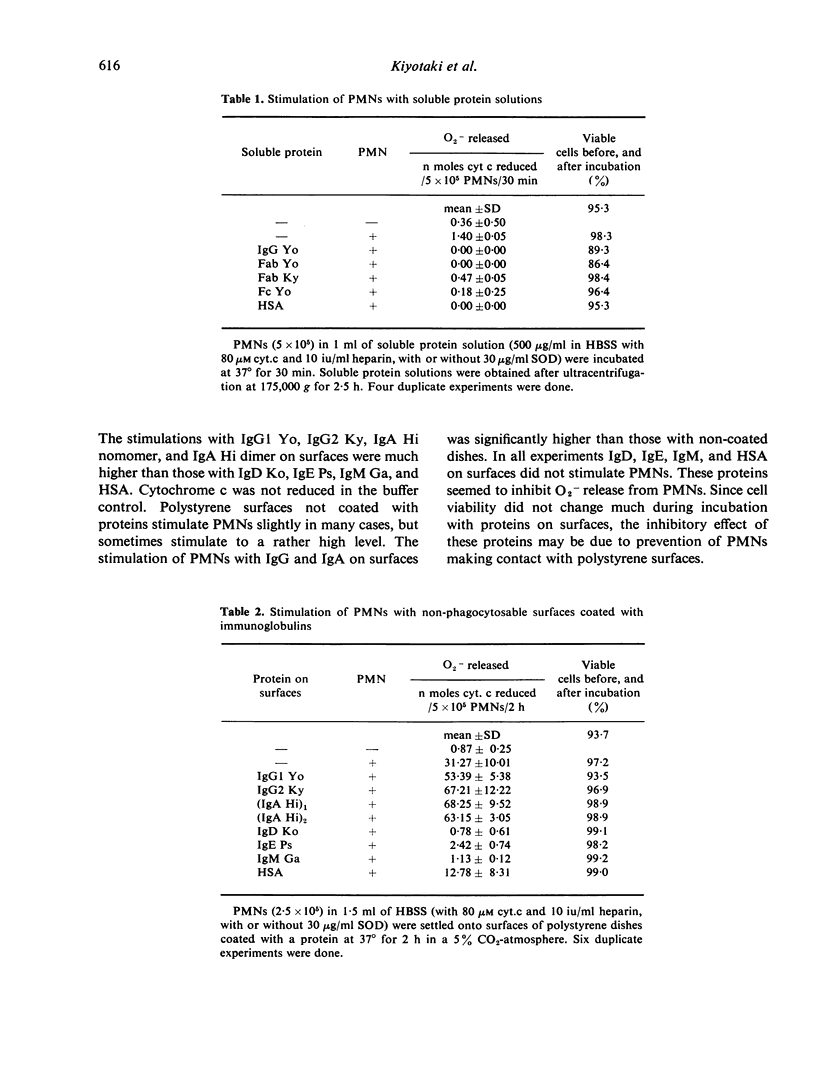
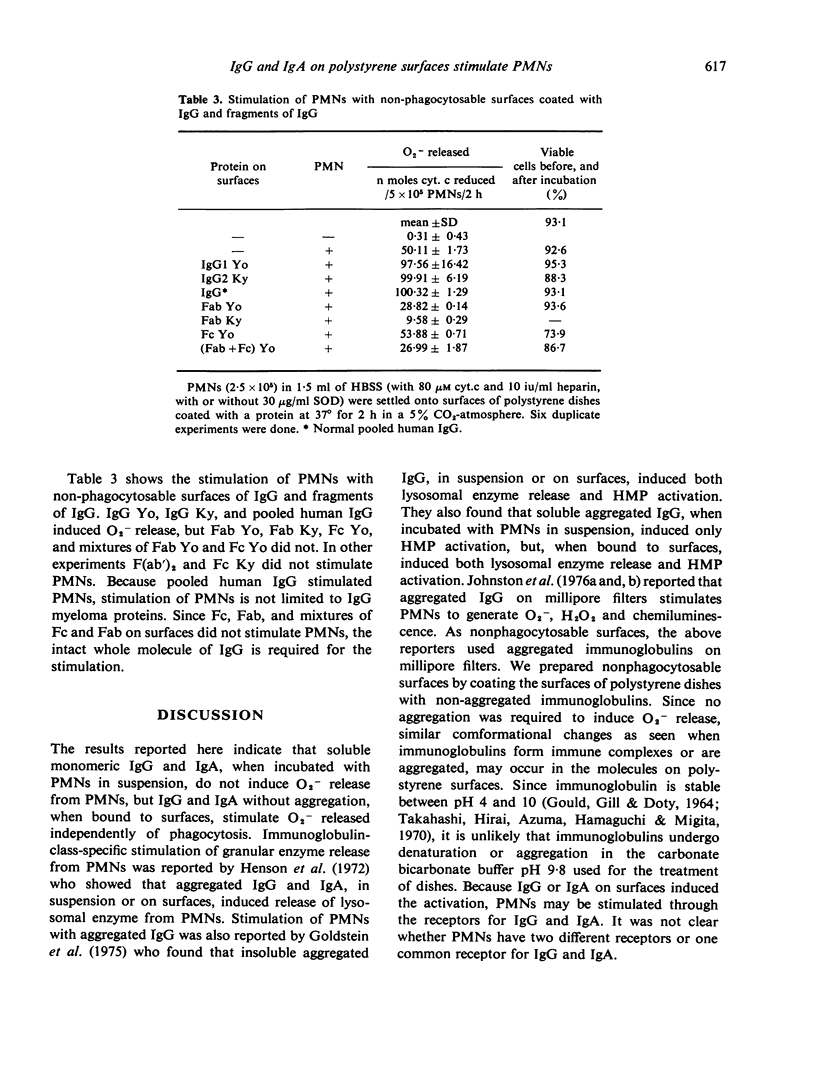
Polystyrene surfaces coated with proteins at alkaline pH were found to be useful to investigate the stimulation of neutrophils independently of phagocytosis. Human neutrophils, when exposed to appropriate stimuli, release superoxide anion (O2-). We have measured superoxide dismutase-inhibitable cytochrome c reduction by cells stimulated with various kinds of protein coated on polystyrene dishes. Native immunoglobulins in solution did not stimulate O2- generation in neutrophils. IgG and IgA adherent to polystyrene dishes stimulated O2- generation in neutrophils, but IgM, IgD, IgE, albumin, Fab, and Fc of IgG on polystyrene dishes did not.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catt K., Tregear G. W. Solid-phase radioimmunoassay in antibody-coated tubes. Science. 1967 Dec 22;158(3808):1570–1572. doi: 10.1126/science.158.3808.1570. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULD H. J., GILL T. J., 3rd, DOTY P. THE CONFORMATION AND HYDROGEN ION EQUILIBRIUM OF NORMAL RABBIT GAMMA-GLOBULIN. J Biol Chem. 1964 Sep;239:2842–2851. [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Radin A., Frosch M. Independent effects of IgG and complement upon human polymorphonuclear leukocyte function. J Immunol. 1976 Oct;117(4):1282–1287. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J Clin Invest. 1975 Oct;56(4):1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T., Lee E. H. Biologic function of the Fc fragments of E myeloma protein. Immunochemistry. 1970 Aug;7(8):687–702. doi: 10.1016/0019-2791(70)90175-8. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani B. Different roles of IgG and complement receptors in phagocytosis by polymorphonuclear leukocytes. J Immunol. 1975 Jul;115(1):15–17. [PubMed] [Google Scholar]
- Messner R. P., Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970 Dec;49(12):2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Watanabe S., Yamamura Y., Putnam F. W. Tryptic digestion of immunoglobulin M in urea: conformational lability of the middle part of the IgM molecule. Immunochemistry. 1974 Nov;11(11):719–727. doi: 10.1016/0019-2791(74)90271-7. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., Prahl J. W., Grey H. M. Structural studies of human gamma D myeloma protein. Biochemistry. 1970 May 12;9(10):2115–2122. doi: 10.1021/bi00812a013. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Hirai T., Azuma T., Hamaguchi K., Migita S. pH-dependent conformation change of a Bence Jones protein. J Biochem. 1970 Jun;67(6):795–799. doi: 10.1093/oxfordjournals.jbchem.a129311. [DOI] [PubMed] [Google Scholar]
- Turner M. W., Bennich H. H., Natvig J. B. Pepsin digestion of human G-myeloma proteins of different subclasses. I. The characteristic features of pepsin cleavage as a function of time. Clin Exp Immunol. 1970 Nov;7(5):603–625. [PMC free article] [PubMed] [Google Scholar]


