Abstract
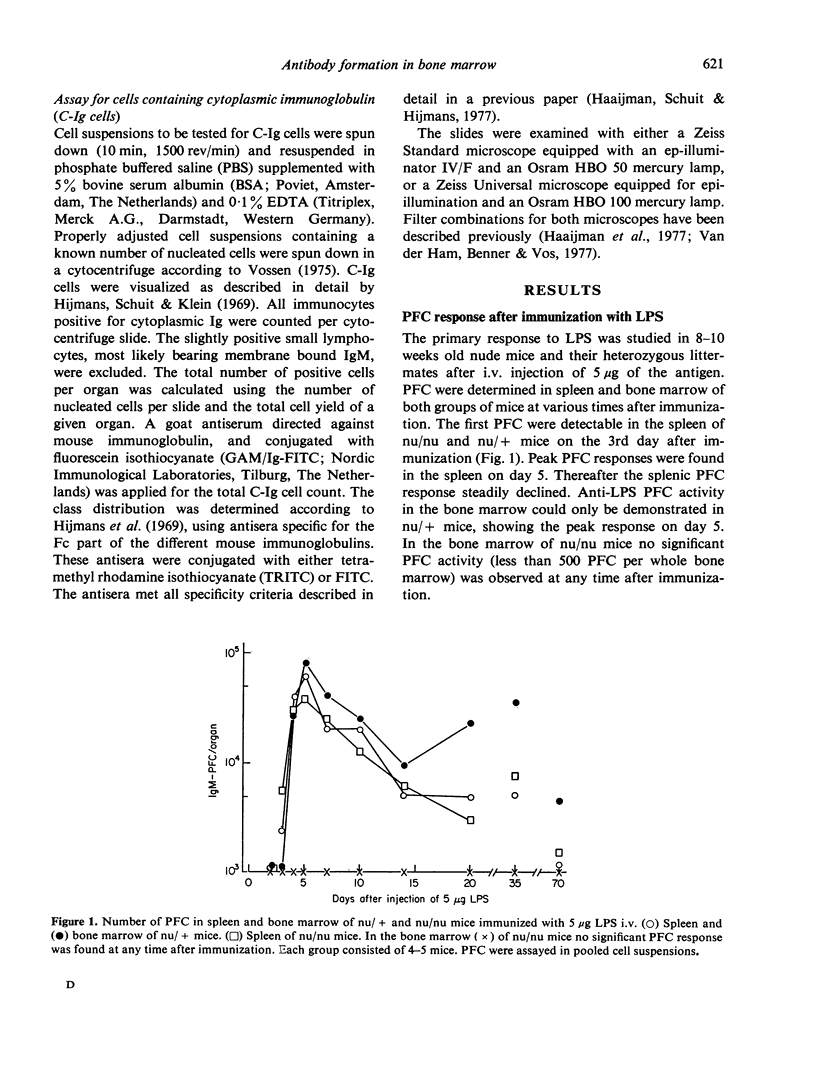
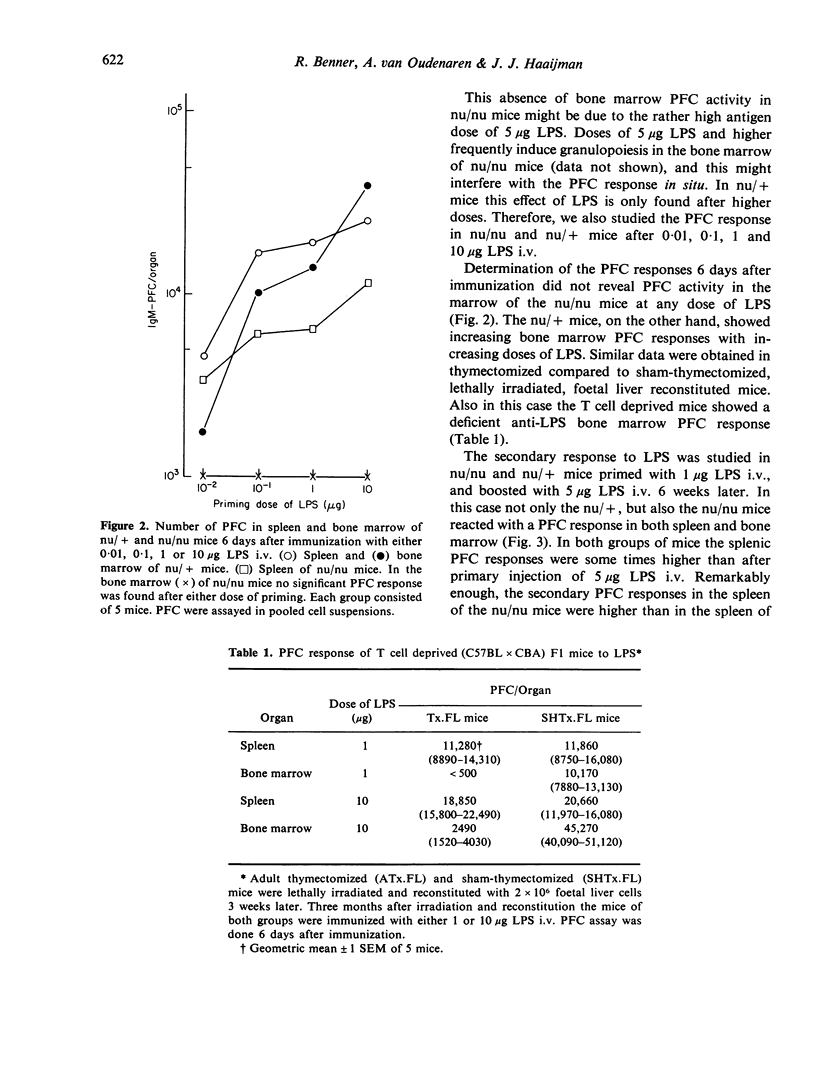
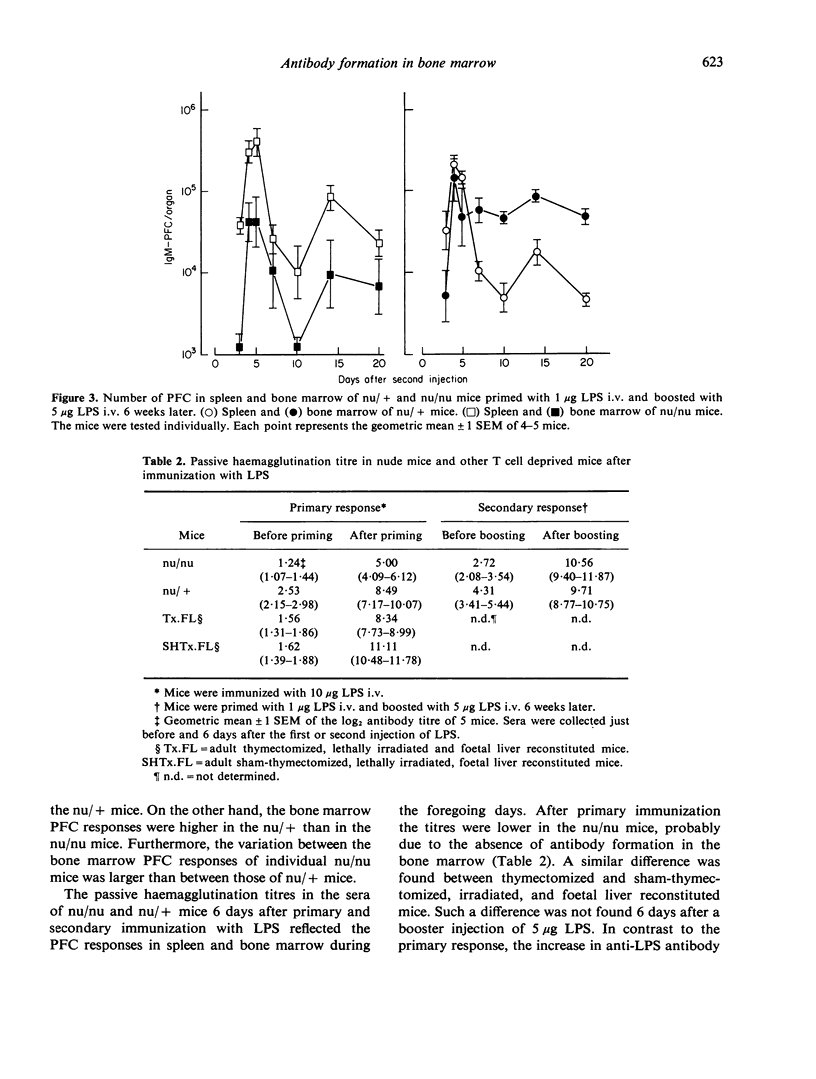
The bone marrow of young adult nude mice was investigated as a site of antibody formation after intravenous immunization with the thymus-independent antigen Escherichia coli lipopolysaccharide (LPS). Mice heterozygous for the nu-gene were found to be capable of a plaque-forming cell (PFC) response in both spleen and bone marrow after primary and secondary immunization with LPS. Primary immunization of nude mice with LPS induced a normal PFC response in the spleen, but did not evoke the appearance of PFC in the bone marrow. During the secondary response the nude mice did show PFC activity in the bone marrow, but at a much lower level than their heterozygous littermates. At all time points after secondary immunization the number of splenic PFC was higher in nude mice than in the control mice.
Determination by immunofluorescence of cells containing cytoplasmic immunoglobulin (C-Ig cells) in the bone marrow of young adult nonimmune nude and heterozygous mice, revealed a three times higher number of C-IgM cells in the bone marrow of the heterozygous thymus-bearing mice. On the other hand, the number of splenic C-IgM cells was higher in the nude mice than in their heterozygous littermates. These results suggest that the presence of the thymus facilitates the appearance of mature antibody-forming cells in the bone marrow of young adult mice, irrespective of whether the generation of these cells is initiated by so called thymus-dependent or thymus-independent antigens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. R., Dresser D. W. The long-term distribution of antibody-forming cells. Eur J Immunol. 1972 Oct;2(5):410–413. doi: 10.1002/eji.1830020505. [DOI] [PubMed] [Google Scholar]
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. I. Regulatory influence of bacterial lipopolysaccharide (LPS) on specific T-cell helper function. J Exp Med. 1974 Jan 1;139(1):24–43. doi: 10.1084/jem.139.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Benner R., Meima F., Van der Meulen G. M., van Ewijk W. Antibody formation in mouse bone marrow. III. Effects of route of priming and antigen dose. Immunology. 1974 Nov;27(5):747–760. [PMC free article] [PubMed] [Google Scholar]
- Benner R., Meima F., van der Meulen G. M., van Muiswinkel W. B. Antibody formation in mouse bone marrow. I. Evidence for the development of plaque-forming cells in situ. Immunology. 1974 Feb;26(2):247–255. [PMC free article] [PubMed] [Google Scholar]
- Benner R., Van Oudenaren A. Antibody formation in mouse bone marrow. VI. The regulating influence of the spleen on the bone marrow plaque-forming cell response to Escherichia coli lipopolysaccharide. Immunology. 1977 Apr;32(4):513–519. [PMC free article] [PubMed] [Google Scholar]
- Benner R., van Oudenaren A., de Ruiter H. Antibody formation in mouse bone marrow. IX. Peripheral lymphoid organs are involved in the initiation of bone marrow antibody formation. Cell Immunol. 1977 Nov;34(1):125–137. doi: 10.1016/0008-8749(77)90235-0. [DOI] [PubMed] [Google Scholar]
- Bloemmen J., Eyssen H. Immunoglobulin levels of sera of genetically thymusless (nude) mice. Eur J Immunol. 1973 Feb;3(2):117–118. doi: 10.1002/eji.1830030213. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H. Secondary IgG responses to type III pneumococcal polysaccharide. II. Different cellular requirements for induction and elicitation. J Immunol. 1976 Apr;116(4):904–910. [PubMed] [Google Scholar]
- Chaperon E. A., Selner J. C., Claman H. N. Migration of antibody-forming cells and antigen-sensitive precursors between spleen, thymus and bone marrow. Immunology. 1968 Apr;14(4):553–561. [PMC free article] [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R., Marsh J. C., Cartwright G. E., Wintrobe M. M. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968 Aug;215(2):353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Thymus-derived lymphocytes sequestered in the bone marrow of hydrocortisone-treated mice. J Immunol. 1972 Mar;108(3):841–844. [PubMed] [Google Scholar]
- Eidinger D., Pross H. F. The immune response to sheep erythrocytes in the mouse. I. A study of the immunological events utilizing the plaque technique. J Exp Med. 1967 Jul 1;126(1):15–33. doi: 10.1084/jem.126.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaijman J. J., Schuit H. R., Hijmans W. Immunoglobulin-containing cells in different lymphoid organs of the CBA mouse during its life-span. Immunology. 1977 Apr;32(4):427–434. [PMC free article] [PubMed] [Google Scholar]
- Hijmans W., Schuit H. R., Klein F. An immunofluorescence procedure for the detection of intracellular immunoglobulins. Clin Exp Immunol. 1969 Apr;4(4):457–472. [PMC free article] [PubMed] [Google Scholar]
- Hill S. W. Distribution of plaque-forming cells in the mouse for a protein antigen. Evidence for highly active parathymic lymph nodes following intraperitoneal injection of hen lysozyme. Immunology. 1976 Jun;30(6):895–906. [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F., Billings P., Cohn M. Functional characteristics of Peyer's patch lymphoid cells. II. Lipopolysaccharide is thymus dependent. J Exp Med. 1974 Feb 1;139(2):407–413. doi: 10.1084/jem.139.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb C., Di Pauli R., Weiler E. Induction of IgG in young nude mice by lipid A or thymus grafts. J Exp Med. 1976 Oct 1;144(4):1031–1036. doi: 10.1084/jem.144.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellbye O. J. Antibody-producing cells in bone marrow and other lymphoid tissues during the primary immune response in mice. Int Arch Allergy Appl Immunol. 1971;40(2):248–255. doi: 10.1159/000230409. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Caporale L. H., Thorbecke G. J. Kinetics of B cell memory development during a thymus "independent" immune response. Cell Immunol. 1974 Jan;10(1):105–116. doi: 10.1016/0008-8749(74)90155-5. [DOI] [PubMed] [Google Scholar]
- Möller G., Michael G. Frequency of antigen-sensitive cells to thymus-independent antigens. Cell Immunol. 1971 Aug;2(4):309–316. doi: 10.1016/0008-8749(71)90065-7. [DOI] [PubMed] [Google Scholar]
- Ness D. B., Smith S., Talcott J. A., Grumet F. C. T cell requirements for the expression of the lipopolysaccharide adjuvant effect in vivo: evidence for a T cell-dependent and a T cell-independent mode of action. Eur J Immunol. 1976 Sep;6(9):650–654. doi: 10.1002/eji.1830060911. [DOI] [PubMed] [Google Scholar]
- Okumura K., Metzler C. M., Tsu T. T., Herzenberg L. A., Herzenberg L. A. Two stages of B-cell memory development with different T-cell requirements. J Exp Med. 1976 Aug 1;144(2):345–357. doi: 10.1084/jem.144.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard H., Riddaway J., Micklem H. S. Immune responses in congenitally thymus-less mice. II. Quantitative studies of serum immunoglobulins, the antibody response to sheep erythrocytes, and the effect of thymus allografting. Clin Exp Immunol. 1973 Jan;13(1):125–138. [PMC free article] [PubMed] [Google Scholar]
- Rozing J., Brons N. H., Benner R. B-Lymphocyte differentiation in lethally irradiated and reconstituted mice. II. Recovery of humoral immune responsiveness. Cell Immunol. 1977 Mar 1;29(1):37–53. doi: 10.1016/0008-8749(77)90273-8. [DOI] [PubMed] [Google Scholar]
- Schrader J. W. The role of T cells in IgG production; thymus-dependent antigens induce B cell memory in the absence of T cells. J Immunol. 1975 Jun;114(6):1665–1669. [PubMed] [Google Scholar]
- Scibienski R. J., Gershwin M. E. Immunologic properties of protein-lipopolysaccharide complexes. I. Antibody response of normal, thymectomized, and nude mice to a lysozyme-lipopolysaccharide complex. J Immunol. 1977 Aug;119(2):504–509. [PubMed] [Google Scholar]
- Von Eschen K. B., Rudbach J. A. Antibody responses of mice to alkaline detoxifield lipopolysaccharide. J Immunol. 1976 Jan;116(1):8–11. [PubMed] [Google Scholar]
- van Muiswinkel W. B., van Soest P. L. Thymus dependence of the IgA response to sheep erythrocytes. Immunology. 1975 Feb;28(2):287–291. [PMC free article] [PubMed] [Google Scholar]
- van der Ham A. C., Benner R., Vos O. Mobilization of B and T lymphocytes and haemopoietic stem cells by polymethacrylic acid and dextran sulphate. Cell Tissue Kinet. 1977 Jul;10(4):387–397. doi: 10.1111/j.1365-2184.1977.tb00306.x. [DOI] [PubMed] [Google Scholar]


