Abstract
Genetic analysis of autoimmune insulin-dependent diabetes mellitus (IDDM) has focused on genes controlling immune functions, with little investigation of innate susceptibility determinants expressed at the level of target β cells. The Alloxan (AL) Resistant (R) Leiter (Lt) mouse strain, closely related to the IDDM-prone nonobese diabetic (NOD)/Lt strain, demonstrates the importance of such determinants. ALR mice are unusual in their high constitutive expression of molecules associated with dissipation of free-radical stress systemically and at the β-cell level. ALR islets were found to be remarkably resistant to two different combinations of β-cytotoxic cytokines (IL-1β, tumor necrosis factor α, and IFN-γ) that destroyed islets from the related NOD and alloxan-susceptible strains. The close MHC relatedness between the NOD and ALR strains (H2-Kd and H2-Ag7 identical) allowed us to examine whether ALR islet cells could survive autoimmune destruction by NOD-derived Kd-restricted diabetogenic cytotoxic T lymphocyte clones (AI4 and the insulin-reactive G9C8 clones). Both clones killed islet cells from all Kd-expressing strains except ALR. ALR resistance to diabetogenic immune systems was determined in vivo by means of adoptive transfer of the G9C8 clone or by chimerizing lethally irradiated ALR or reciprocal (ALR × NOD)F1 recipients with NOD bone marrow. In all in vivo systems, ALR and F1 female recipients of NOD marrow remained IDDM free; in contrast, all of the NOD recipients became diabetic. In conclusion, the ALR mouse presents a unique opportunity to identify dominant IDDM resistance determinants expressed at the β cell level.
Keywords: diabetes, cytokines, free radicals, pancreatic islets, T cells
The emphasis of studies of the genetic basis for resistance or susceptibility to autoimmune insulin-dependent diabetes mellitus (IDDM) has focused on genes associated with immunoregulation. Many insights into such immunoregulatory genes have come from studies using IDDM-susceptible nonobese diabetic (NOD) mice (1). Results from islet transplantation experiments have suggested that β cells are passive targets, having little role in the pathogenic process other than presentation of the relevant autoantigens. Generation of cytotoxic free radicals is a key pathogenic component in β-cell destruction resulting from the combination of both direct (cytotoxic T-cell-mediated) and indirect (cytokine-, nitric oxide- or free-radical-mediated) events. Stress-response genes are involved in both cellular defense and repair of cells after damage. Several reports have determined that stress-response genes (including antioxidant enzymes) either are not expressed or are present at very low levels in β cells (2, 3). This lack of cellular protection could contribute to the demise of β cells in IDDM. Recently, we reported that a close relative of NOD mice, the Alloxan (AL) Resistant (R)/Leiter (Lt) strain, maintains an unusual constitutive increase in a battery of cellular defenses, both systemically and at the islet level (4). These parameters included elevated pancreatic total superoxide dismutase, glutathione (GSH) reductase, and GSH peroxidase as well as an increase in the reduced-to-oxidized GSH ratio. Islets of the ALR strain have been shown to be resistant in vivo to the free-radical generating diabetogens alloxan and streptozocin (5, 6). Studies in vitro demonstrated that ALR islets maintained glucose-stimulated insulin secretion (GSIS) and viability even after culture with powerful oxidizing agents that were cytotoxic to ALS/Lt islets (6).
IDDM-prone NOD mice and the resistant ALR strain both share a common origin from outbred Institute for Cancer mice. Our genome-wide scans have confirmed a high degree of genetic relatedness between these two strains. Indeed, the MHCs of these two strains are quite similar (7). The H2gx MHC haplotype of ALR mice is identical to the NOD H2g7 haplotype at the Kd class I allele and through the entire class II region. However, the distal regions of the H2gx and H2g7 haplotypes differ, respectively, including a Ddx vs. a Db MHC class I variant. Furthermore, the H2gx haplotype of the ALR strain was shown recently to be identical to the MHC of the Cataract Shinogi (CTS/Shi) strain (8). When the H2gx of CTS/Shi was moved congenically onto the NOD background, IDDM onset was delayed, and overall incidence was decreased relative to H2g7 homozygous segregants (9). Nevertheless, the fact that some level of IDDM still developed in NOD.CTS-H2gx mice indicated that this haplotype promotes diabetogenesis. Furthermore, simple sequence-repeat markers indicated considerable genetic identity between ALR and NOD genomes in regions containing most of the known NOD-derived insulin-dependent diabetes (Idd) susceptibility loci. Hence, it might have been predicted that the ALR strain would also be susceptible to autoimmune IDDM induction if exposed to a NOD-immune system. In this report, we extend a previous finding that ALR mice are resistant to chemical diabetogenesis by demonstrating that they also are resistant to β-cell killing by combined cytokines, by diabetogenic NOD islet-reactive cytotoxic T lymphocytes (CTLs), and by bone marrow reconstitution of a NOD-immune system.
Materials and Methods
Mice.
ALR/Lt, Alloxan-susceptible (ALS)/Lt (H2nb1), CTS/ Shi, NOD/Lt, NOD.nonobese nondiabetic (NON)-H2nb1/Lt (NOD.H2nb1), NOD.AI4αβ-transgenic/Sr (NOD.AI4) (7), NOD.Prkdcscid/LtSz [NOD.severe combined immunodeficiency (scid)], NON.NOD-H2g7 (NON.H2g7), and the NOD-related diabetes-resistant (NOR)/Lt mice used in this study were bred and maintained in the specific pathogen-free research animal facility at The Jackson Laboratory. All mice were allowed free access to food [autoclaved diet NIH-31, 6% fat (Purina)] and acidified drinking water.
Islet Isolation.
Pancreatic islets from female mice were isolated by using a published collagenase inflation method (10). Islets were hand picked for purity in Hanks' balanced salt solution under a dissecting microscope after isolation on a Histopaque 1119 (Sigma) gradient.
Culture of Islets with Combined Cytokines.
Islets were cultured overnight and then treated for 15 h in either control DMEM (Life Technologies) supplemented with 10 units/ml gentamycin (Sigma) and 10% (vol/vol) heat-inactivated FBS (Atlanta Biologicals, Norcross, GA), or in medium plus the combinations of 5 units/ml IL-1β, 100 units/ml tumor necrosis factor α (TNFα), and 100 units/ml IFN-γ or 10 units/ml IL-1β, 500 units/ml TNFα, and 500 units/ml IFN-γ. Recombinant mouse TNFα and IL-1β were obtained from PeproTech (Rocky Hill, NJ), and rat-recombinant IFN-γ was a kind gift of P. van de Meide, (Biomedical Primate Research Center, Rijswijk, The Netherlands).
Determination of Islet Insulin-Secretory Capacity.
Islet GSIS capacity and insulin content of cytokine-treated islets were assayed by using a modified protocol described by Eizirik (11). Briefly, islets were exposed sequentially to 2.8 mM glucose for 1 h followed by 22 mM glucose for 2 h. GSIS values were reported as the difference between media insulin concentration for 22 mM vs. 2.8 mM. This value was normalized by islet DNA concentrations determined fluorometrically (DNA Fluorometer TKO 100, Hoefer) by using Hoechst 33258 dye (Polysciences), with herring sperm DNA (Promega) as a standard.
Determination of NOD-Derived Diabetogenic CTL Reactivity Against Target β Cells.
Cell-mediated lysis (CML) assays were performed as described (12, 13). Two NOD-derived H2-Kd-restricted β-cell autoreactive CD8+ CTLs, AI4 (13) and G9C8 (14) were used. NOD.AI4αβ-transgenic mice transgenically expressing the T-cell antigen receptor (Vα8/Vβ2) of the diabetogenic AI4 T cell clone are as described (15). Freshly isolated splenic AI4 T cells were harvested from these mice. They then were activated in vitro as described (13). The insulin Bchain peptide- (amino acids 15–23) reactive G9C8 clonal line was provided kindly by Susan Wong (Yale University, New Haven, CT). For CML, single-cell suspensions prepared from pancreatic islets were labeled with 51Cr (ICN) for 60 min at 37°C. Labeled islet cells were placed in a 96-well flat-bottom microtiter plate (5,000 cells per well) along with AI4 or G9C8 T cells at effector-to-target ratios of 1:1, 10:1, and 50:1, in 200 μl of DMEM (Life Technologies) supplemented with 50 mg/ml gentamycin/5 × 10−5 M 2-mercaptoethanol/7.4 mM Hepes/13.6 μM folic acid/272.5 μM l-asparagine/0.56 mM l-arginine/1 mM sodium pyruvate/1.4 mM l-glutamine/5% (vol/vol) FBS. Specific lysis of targets by AI4 and G9C8 was determined by gamma counting 100 μl of media after a 16-h incubation at 37°C (Wallac, Turku, Finland).
Flow-Cytometric Comparison of NOD, NOR, and ALR Islet Cell MHC Class I Surface Expression.
Islets were isolated and single cells were prepared as described above. Levels of expression of the MHC class I H2-Kd molecule common to the ALR, NOD, and NOR strains were assessed in triplicate on pooled samples of islet cells by flow cytometry (FACScan, Becton Dickinson) with the FITC-conjugated monoclonal antibody SF1–1.1 (7).
Assessment of IFN-γ Production by NOD-Derived Diabetogenic CTLs.
Islets were isolated as described above. Incubations were performed in 96-well plates at 37°C in a humidified incubator with a 95% air/5% CO2 atmosphere. Wells contained: AI4 or G9C8 T cells only; islets only; or islets and T cells in 200 μl of the DMEM mixture described above. Plates were incubated for 72 h, at which time 100 μl of the medium was removed and assessed for IFN-γ concentration by ELISA (OptEIA mouse IFN-γ, PharMingen).
In Vivo Studies to Determine Islet Susceptibility to Diabetogenic T Cells.
Female NOD, ALR, reciprocal (ALR × NOD)F1 and (NOD × ALR)F1 mice were irradiated lethally at 4 weeks of age with 1,200 rad from a Cs137 source and then reconstituted as described (16) with 5 × 106 T cell-depleted bone marrow cells isolated from either standard NOD or NOD.AI4 females. These bone-marrow chimeras then were monitored weekly for glycosuria development (Diastix, a kind gift of Bayer, Elkhart, IN) for the next 20 weeks. Diabetes was scored as two sequential positive tests. After diabetes development or at the end point, peripheral blood and splenic leukocytes were stained with appropriate monoclonal antibodies and typed by flow cytometry to determine reconstitution of B cells [B220-phycoerythrin (PE), Ig-FITC], macrophages (Mac1-PE), granulocytes (Gr1-FITC), T cells (CD3-FITC, CD4-Cy3, and CD8-PE), and for the Class I H2-D allele present (28–24-8-FITC identifying the NOD Db allele and 34–2-12-FITC cross-reactive with the Ddx allele of ALR). The presence of AI4 transgenic T cells in recipients of marrow from T-cell antigen receptor transgenic donors was detected by Vα8-PE, Vβ2-FITC double staining. At diabetes onset or at the end of the indicated observation period, pancreata were removed for histological examination and scored for an insulitis index (17) with values ranging between 0 (no lesions) and 1.0 (end-stage islets).
Diabetogenicity of the G9C8 T cell clone maintained in coculture with standard NOD.scid islets in DMEM containing 5% (vol/vol) FBS, 50 mg/ml gentamycin, and 5 units/ml IL-2 (PeproTech) was determined in an adoptive-transfer system. G9C8 cells (107) were injected i.v. (retroorbital sinus) into sublethally irradiated (725 rad) males. Diabetes development was monitored by glycosuria on a daily basis for 20 days after the transfer. Pancreata from diabetic mice or from mice that remained normoglycemic through 20 days after the transfer were histologically evaluated for insulitis (17).
Assessment of Susceptibility to Cyclophosphamide (CYP)-Induced Diabetes.
Eight-week-old ALR and NOD males and females (five per strain per sex) were screened for susceptibility to CYP-induced IDDM. Mice received two injections i.p. of 200 mg/kg body weight of CYP (Sigma) spaced 2 weeks apart. Controls received 2 injections of PBS vehicle. Mice were tested over an ensuing 8-week period for development of glycosuria by using Diastix.
Statistical Analyses.
All values reported are means ± SD. Significance was determined with a one-way ANOVA by using SUPERANOVA for the Macintosh (Abacus Concepts, Berkeley, CA).
Results
ALR/Lt Islets Exhibit Strong Resistance to Proinflammatory Cytokines in Vitro.
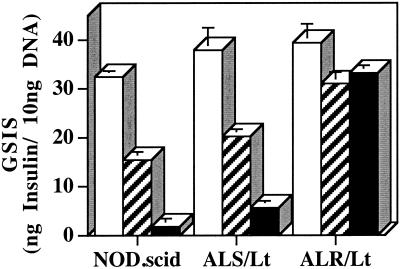
The effects of the combination of IL-1β, TNFα, and IFN-γ in vitro are clearly detrimental to human, rat, and mouse islet structure, function, and viability (18). In contrast to the marked reduction in GSIS mediated by both levels of combined cytokines in islets from NOD.scid and ALS donors, ALR islets were remarkable in showing a nonsignificant reduction in GSIS (Fig. 1). Islets of the NOD.scid and ALS strains exposed for 15 h to 100 units/ml of IFN-γ, 100 units/ml TNFα, and 5 units/ml IL-1β exhibited GSIS reductions of 52 and 47%, respectively. When exposed to a higher concentration (500 units/ml IFN-γ, 500 units/ml TNFα, and 10 units/ml IL-1β), insulin secretory capacity of NOD.scid and ALS islets was reduced to only 5.6 and 15% of the untreated controls, respectively. By comparison, GSIS exhibited by ALR islets exposed to both concentrations of combined cytokines was not significantly different from untreated control islets. Visual inspection by phase-contrast microscopy showed that ALR islets maintained structural integrity in both concentrations of combined cytokines, whereas islets from NOD.scid and ALS were disintegrating. Whereas β cell death mediated by combinations of proinflammatory cytokines has been associated with both necrotic and apoptotic mechanisms, both mechanisms share the production of toxic free radicals. That ALR islets are resistant to this potent proinflammatory stress is further evidence of their unusually elevated defense against free-radical mediated damage.
Figure 1.
Ability of islets to secrete insulin after treatment with proinflammatory cytokines. Isolated islets from the NOD.scid, ALS/Lt, and ALR/Lt strains were incubated for 15 h in 5.5 mmol glucose DMEM (open bars) or 5.5 mmol glucose DMEM containing 5 units/ml IL-1β, 100 units/ml TNFα, and 100 units/ml IFN-γ (hatched bars) or 10 units/ml IL-1β, 500 units/ml TNFα, and 500 units/ml IFNγ (solid bars). Islets were washed free of the cytokine incubation media and assayed for GSIS in 22 vs. 2.8 mmol glucose DMEM. Data represent the mean difference between basal and GSIS ± SEM. Results are calculated from two independent experiments performed in triplicate.
ALR/Lt Islets Exhibit Complete Resistance to Lysis by Islet-Reactive CTL in Vitro.
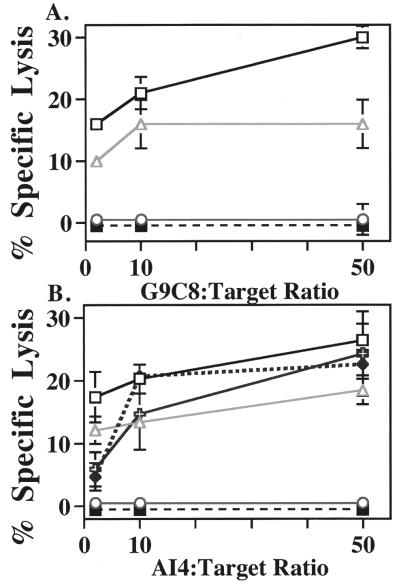
Because ALR and NOD share a common H2-Kd allele, we tested susceptibility of ALR islet cells to lysis by H2-Kd-restricted diabetogenic CD8+ T cell clones derived from NOD (Fig. 2A). At all effector-to-target ratios, the G9C8 CTL clone lysed NOD.scid and CTS islet cells [the latter is an important control because of H2gx identity with ALR (7)]. In contrast, ALR islet cells were resistant completely to lysis by the G9C8 CTL clone. The H2-Kb (negative control) islets from the NOD.H2nb1 congenic stock were not lysed. Cell-mediated lysis data in Fig. 2B show that this resistance was not limited to the insulin peptide-reactive G9C8 clone but was extended to killing by AI4 CTL. With the exception of the ALR islet cells, all Kd-expressing strains tested (NOD.scid, CTS, NOR, and NON.H2g7) were lysed by the AI4 CTL at all effector-to-target ratios. Again, the H2-Kb expressing NOD.H2nb1 islet cells were not lysed, demonstrating restriction of AI4 and G9C8 CTLs to Kd as opposed to Db MHC class I molecules (the latter was expressed both by susceptible NOD and resistant NOD.H2nb1 islets).
Figure 2.
Ability of the NOD-derived Kd-restricted G9C8 (A) or AI4 (B) CD8+ T-cell clones to specifically lyse dispersed islet cells isolated from the Kd-expressing NOD.scid (▫), CTS/Shi (▵), NOR/Lt ( ), NON.H2 g7 (♦), and ALR/Lt (○) mice and the Kb-expressing NOD.H2nb1 (▪) congenic strain (negative control). Islets were isolated, dispersed into single cells, labeled with 51Cr and incubated with T-cell clones for 16 h, and specific lysis was determined. Data represent the mean percentage specific cytotoxicity of two independent experiments performed in triplicate.
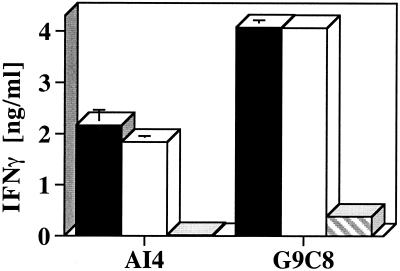
Conceivably, ALR β-cell resistance might be explained by low cell-surface H2-Kd expression compared with β cells from other strains. FACS analysis with the Kd-specific SF1–1.1-FITC-conjugated antibody demonstrated equivalent Kd expression on CTL-resistant ALR [mean fluorescence intensity (MFI) = 471.3 ± 19.2] vs. either the susceptible NOR (MFI = 475.1 ± 11.1) or NOD.scid (MFI = 483.4 ± 14.3) islet cells. To determine whether the CTLs were activated by means of contact with ALR islet cells, IFN-γ secretion by G9C8 and AI4 T cells in the absence and presence of NOD and ALR islet cells was measured (Fig. 3). Analysis of supernatants from G9C8 and AI4 cells exposed to islets for 72 h showed that both islet sources activated both populations of CTL. Islets isolated from NOD.H2nb1 mice (negative control, data not shown) failed to stimulate IFN-γ production from either T-cell population. To determine whether NOD-immune cells can recognize and destroy nonislet tissue from ALR mice, reciprocal skin grafts were performed between NOD and ALR or between NOD and ALS (the latter being completely MHC disparate). NOD rejected the completely allogeneic ALS skin within 7 days and the semiallogenic ALR skin by 21 days. This clearly demonstrates that NOD T cells are not blind to ALR alloantigens.
Figure 3.
Activation of CTL after islet exposure. Secretion of IFN-γ from NOD-derived Kd-restricted CD8+ T cells after 72-h exposure to isolated islets from the Kd-expressing NOD.scid (solid bars) and ALR/Lt (open bars) strains. Hatched bars represent IFN-γ released from unstimulated CTL. Data represent the mean ± SD of two experiments.
ALR/Lt Mice Exhibit Complete Resistance to IDDM Mediated by Diabetogenic Effectors in Vivo.
The unusual resistance exhibited by ALR islets to lysis by either combined cytokines or NOD CTL in vitro was extended to resistance of ALR pancreatic islets in situ. Resistance in vivo to the G9C8 clone was assessed by adoptive transfer. Whereas IDDM developed in 100% of NOD recipients by 8 days after transfer, ALR mice remained completely resistant 20 days after transfer (Table 1). As expected, histological examination revealed total destruction of the endocrine pancreas in all NOD mice receiving the insulin peptide-reactive G9C8 CTL (insulitis index, 1.0). No insulitis was present in the ALR or F1 mice receiving the G9C8 cells (insulitis index, 0).
Table 1.
Determination of the diabetogenicity of the insulin-reactive G9C8 CTL clone
| Recipient 107 cells | n | IDDM 10 days after transfer, % | Insulitis index (1 = max) |
|---|---|---|---|
| NOD | 6 | 100 | 1.00 |
| F1 | 6 | 0 | 0 |
| ALR | 6 | 0 | 0 |
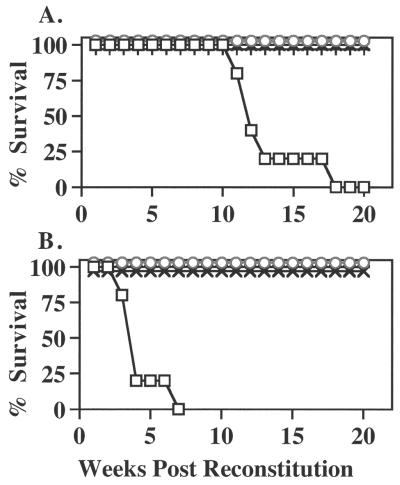
Transplantation of NOD marrow into irradiated NOD female recipients elicited IDDM in 80% by 12 weeks and 100% by 18 weeks after reconstitution (Table 2; Fig. 4A). Histological examination was consistent with the diabetes phenotype, showing complete destruction of the endocrine pancreas. In sharp contrast, ALR and F1 mice from reciprocal outcrosses were resistant to IDDM induction by NOD bone marrow. The low insulitis indices in ALR and F1 recipients represented the detection of a low frequency of periinsulitis (Table 2). Flow-cytometric analysis of T cell populations in these bone marrow chimeras documented equal reconstitution of a NOD-immune repertoire in both the IDDM-susceptible NOD and the resistant ALR and reciprocal F1 recipients (Table 2). In these latter groups, determination of NOD class I H2-Db vs. ALR H2-Ddx expression confirmed that the ablation of the recipient's hematopoietic system was complete, as Ddx was not detectable in the ALR or F1 chimeras (Table 2). NOD recipients of ALR bone marrow failed to develop IDDM, and insulitis was absent (Table 2, Fig. 4A). Interestingly, the repopulating ability of the ALR marrow was different significantly in NOD vs. ALR recipients. Flow-cytometric profiles of the spleen demonstrate that ALR marrow generated significantly higher percentages of peripheral T cells when maturing in the NOD compared with ALR hosts (Table 2).
Table 2.
The ALR genome provides dominant protection from the adoptive transfer of diabetes by NOD bone marrow
| Marrow donor | Recipient (1,200 rad treated) | IDDM 20 weeks after reconstitution, % | Insulitis index (max = 1) | Splenocytes, %
|
||||
|---|---|---|---|---|---|---|---|---|
| CD8+ | CD4+ | CD3+ | Db | Ddx | ||||
| NOD | NOD | 100 | 1.00 | 10.2 ± 2* | 27.8 ± 5*† | 38 ± 6* | 100 ± 0 | 0 ± 0 |
| NOD | ALR | 0 | 0.10 | 7.8 ± 2*† | 30.0 ± 3* | 38 ± 4* | 96 ± 2 | 0 ± 0 |
| NOD | (ALR × NOD) F1 | 0 | 0.09 | 8.8 ± 1* | 27.4 ± 1* | 36 ± 1* | 96 ± 1 | 1 ± 1 |
| NOD | (NOD × ALR) F1 | 0 | 0.12 | 9.4 ± 1* | 28.4 ± 1* | 38 ± 1* | 98 ± 1 | 1 ± 1 |
| ALR | ALR | 0 | 0 | 2.9 ± 1‡ | 13.6 ± 1‡ | 17 ± 1‡ | 0 ± 0 | 99 ± 0 |
| ALR | NOD | 0 | 0 | 5.3 ± 1† | 23.0 ± 1† | 28 ± 2† | 2 ± 0 | 98 ± 0 |
Data shown are mean ± SD. n = 5 for all experiments. Means with different symbols (*, †, ‡) within a column are significantly different at P < 0.05. Means with the same symbol are not significantly different.
Figure 4.
IDDM incidence in bone marrow chimeras reconstituted with bone marrow from either NOD/Lt (A) or NOD.AI4αβ-transgenic (B) females. Female NOD/Lt (▫) ALR/Lt (○), as well as reciprocal F1 mice [NOD × ALR (×) and ALR × NOD (×)] were reconstituted at 4 weeks of age. These bone marrow chimeras then were monitored for glycosuria weekly.
An even greater test of ALR resistance to autoimmune IDDM was provided by engraftment with bone marrow from the NOD.AI4 transgenic donors in which IDDM development is accelerated, as evidenced by a 90% disease rate by 8 weeks of age (15). As shown in Table 3 and Fig. 4B, 100% of the NOD recipients of bone marrow cells from NOD.AI4 donors developed IDDM by 7 weeks after transfer. Histologically, all exhibited complete destruction of the endocrine pancreas, and three exhibited pancreatitis. In sharp contrast, ALR and its F1 hybrids chimerized with this highly diabetogenic marrow remained completely IDDM free and exhibited only sporadic and primarily periinsulitis. A higher percentage of total CD8+ T cells [expressing both transgenic and endogenous T cell antigen receptors(TCR)] was seen in NOD vs. ALR recipients of NOD.AI4 marrow (Table 3). However, the ALR host environment selected a higher percentage of Vα8Vβ2 TCR transgene-positive CD8+ cells. Therefore, the ALR resistance was even more remarkable.
Table 3.
The ALR genome provides dominant protection from the adoptive transfer of diabetes by NOD.AI4 bone marrow
| Marrow donor | Recipient (1,200 rad treated) | IDDM 20 weeks reconstitution, % | Insulitis index (1 = max) | Splenocytes, %
|
|||
|---|---|---|---|---|---|---|---|
| CD8+ | CD4+ | TgCD8+ | TgCD4+ | ||||
| NOD.AI4 | NOD | 100 | 1.00 | 15.4 ± 1* | 26.1 ± 3* | 70 ± 5* | 87 ± 6* |
| NOD.AI4 | ALR | 0 | 0.10 | 7.2 ± 1† | 17.2 ± 1† | 99 ± 1† | 62 ± 6† |
| NOD.AI4 | (ALR × NOD)F1 | 0 | 0.15 | 8.6 ± 1† | 19.0 ± 1† | 92 ± 3† | 28 ± 1‡ |
| NOD.AI4 | (NOD × ALR)F1 | 0 | 0.20 | 7.4 ± 2† | 19.0 ± 2† | 72 ± 1* | 21 ± 1§ |
Data shown are mean ± SD. n = 5 for all experiments. Means with different symbols (*, †, ‡) within a column are significantly different at P < 0.05. Means with the same symbol are not significantly different.
ALR Mice Are Resistant to CYP-Induced Diabetes.
In many instances in which the presence of protective alleles or environmental immunomodulation suppresses spontaneous IDDM in NOD mice or NOD hybrid/congenic stocks, two serial doses of CYP can override the resistance. CYP induced IDDM in 100% of the NOD females within 2 weeks after the second injection, and 80% of the CYP-treated NOD males became diabetic during the 8-week period. In contrast, none of the ALR mice became diabetic during the 8-week period after the second CYP injection.
Discussion
ALR mice and their pancreatic islets were found previously to be unusually resistant to killing by chemicals generating oxygen free radicals (4, 6). The studies reported here now demonstrate that ALR β cells also are resistant, remarkably, to killing mediated by exposure to highly β-cytopathic cytokine combinations in vitro. Further, we show both in vitro and in vivo that ALR β cells are resistant to destruction by autoimmune T cells from the NOD mouse. In reciprocal outcross with NOD, the ALR resistance to immune-mediated β-cytotoxic stress was inherited as a dominant trait in F1 progeny. This same dominant inheritance was observed previously for resistance to necrotic damage mediated by alloxan (6). This resistance is remarkable not only because of the extensive allele identity throughout much of the MHC complex, including the class II region, but also because of extensive genetic identity at simple sequence-repeat markers linked to non-MHC “Idd ” loci (unpublished results). This finding has important implications for the genetics of type I diabetes in rodents and in humans. Until now, consideration of potential candidate genes contributing to differential IDDM susceptibility has primarily focused on loci controlling antigen presentation or immune effector or regulatory functions. For example, although IDDM2 in humans is associated with polymorphisms in the variable nucleotide terminal repeat region adjacent to the insulin gene, the favored interpretation has been that its diabetogenic contribution is at the level of tolerance-inducing thymic antigen-presenting cells and not in insulin secretory dynamics at the β cell level (19). The β cell itself is generally viewed as a hapless victim of a dysregulated immune system. In this regard, the ALR strain presents a startling illustration that a genetically determined capacity for efficient dissipation of free-radical stress, expressed both systemically and at the β cell level, can confer unusually strong resistance to development of autoimmune, T-cell-mediated IDDM.
Mice or their β cells experimentally modified to overexpress genes encoding enzymes that diffuse free-radical stress have been described (20–22). Pancreatic β cell overexpression of antioxidant enzymes protects against apoptosis (23–25) and chemically induced diabetes (20, 21), and protects partially from autoimmune diabetes (21). Other members of the stress-response system, such as heat-shock proteins and antiapoptotic molecules, also protect against the toxic effects of proinflammatory cytokines on β cells (22, 26), including decreases in insulin content and secretion, DNA fragmentation, as well as membrane lipid peroxidation (27). The resistance of ALR islets to free-radical-mediated diabetogenic stress is consistent therefore with the ALR strain characteristic systemic increase in antioxidant and stress-response enzyme activity. The present report demonstrates that isolated islets from different inbred mouse strains differ in their ability to diffuse cytopathic injury mediated by cytotoxic combinations of proinflammatory cytokines. Presumably, cell death resulting from exposure to the combinations of IL-1β, TNFα, and IFN-γ tested in the present study entails both necrotic and apoptotic mechanisms (28), processes which also are used by cytotoxic T cells in the destruction of targets.
When the cell-mediated killing mechanisms of the two CTL populations used in this study, the G9C8 and the AI4 CTL, are considered, the ALR islet resistance is even more fascinating because the two CTLs appear to use different cytopathic mechanisms. We have observed that G9C8 cells do not kill NOD islets obtained from Fas-deficient congenic donors (kindly provided by A. Chervonsky, The Jackson Laboratory), whereas AI4 CTLs do kill these Fas-deficient targets. This implies that ALR islets are resistant equally to cell death triggered through activation of apoptotic cascades and the more classical CTL-mediated lytic pathways associated with perforin/granzyme A. Because both necrotic and apoptotic cell death entails generation of reactive oxygen species, we had hypothesized initially that the basis for this “global” resistance of ALR islets, as well as the constitutive high levels of free-radical-dissipating enzymes expressed systemically, was the presence of an up-regulated transcription factor capable of regulating a diverse set of genes with the appropriate stress-response elements (6). However, in a preliminary experiment comparing gene expression profiles from the livers of unmanipulated ALR vs. NOD and ALS mice, multiple transcripts encoding a variety of different transcription factors were elevated significantly in ALR in comparison to the other two strains. Hence, it is equally plausible that an intracellular stress-response factor coupling mRNA transcription to protein translation may be elevated constitutively. The only two transcripts markedly reduced in ALR vs. the two comparison strains encoded two proapoptotic proteins (Apaf1 and Bak). This latter observation may indicate that mitochondria in ALR are more resistant to loss of ATP production and maintenance of intracellular reducing equivalents.
In summary, the molecular basis for the impressive resistance of ALR mice to diabetogenic stresses mediated by multiple pathogenic pathways remains to be established. The finding that resistance appears to be inherited as a dominant phenotype indicates that discovery of the gene or genes contributing to this resistance may have clinical applications. If such genes are differentially expressed in human organs used for transplantation, obviously those organs expressing higher levels might be expected to transplant more successfully. If such genes are not expressed constitutively but can be induced, transplantation outcomes may still be improved. Finally, it may be possible to transduce tissues and organs used in transplantation with such genes. These possibilities all await further elucidation of the genetics of this most interesting mouse strain.
Acknowledgments
This work was supported by the Juvenile Diabetes Foundation International, the American Diabetes Association, and National Institutes of Health Grants DK27722, DK36175, DK46266, DK51090, and AI41469. Institutional shared services were supported by National Cancer Institute Center Support Grant CA-34196 (to T.J.L.).
Abbreviations
- IDDM
insulin-dependent diabetes mellitus
- NOD
nonobese diabetic
- AL
Alloxan
- R
resistant
- Lt
Leiter
- GSIS
glucose-stimulated insulin secretion
- CTS/Shi
Cataract Shinogi
- CTL
cytotoxic T lymphocyte
- ALS
Alloxan-susceptible
- NOR
NOD-related diabetes-resistant
- SCID
severe combined immunodeficient
- TNFα
tumor necrosis factor α
- CYP
cyclophosphamide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Leiter E H. In: NOD Mice and Related Strains: Research Applications in Diabetes, AIDS, Cancer, and Other Diseases. Leiter E H, Atkinson M A, editors. Austin, TX: R. G. Landes; 1998. pp. 37–69. [Google Scholar]
- 2.Grankvist K, Marklund S L, Taljedal I B. Biochem J. 1981;199:393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenzen S, Drinkgern J, Tiedge M. Free Radical Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 4.Mathews C E, Leiter E H. Free Radical Biol Med. 1999;27:449–455. doi: 10.1016/s0891-5849(99)00084-2. [DOI] [PubMed] [Google Scholar]
- 5.Ino T, Kawamoto Y, Sato K, Nishikawa K, Yamada A, Ishibashi K, Sekiguchi F. Jikken Dobutsu. 1991;40:61–67. doi: 10.1538/expanim1978.40.1_61. [DOI] [PubMed] [Google Scholar]
- 6.Mathews C E, Leiter E H. Diabetes. 1999;48:2189–2196. doi: 10.2337/diabetes.48.11.2189. [DOI] [PubMed] [Google Scholar]
- 7.Graser R T, Mathews C E, Leiter E H, Serreze D V. Immunogenetics. 1999;49:722–726. doi: 10.1007/s002510050673. [DOI] [PubMed] [Google Scholar]
- 8.Mathews C E, Graser R T, Serreze D V, Leiter E H. Diabetes. 2000;49:131–134. doi: 10.2337/diabetes.49.1.131. [DOI] [PubMed] [Google Scholar]
- 9.Ikegami H, Makino S, Yamato E, Kawaguchi Y, Ueda H, Sakamoto T, Takekawa K, Ogihara T. J Clin Invest. 1995;96:1936–1942. doi: 10.1172/JCI118239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ablamunits V, Elias D, Cohen I R. Clin Exp Immunol. 1999;115:260–267. doi: 10.1046/j.1365-2249.1999.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eizirik D L, Sandler S, Hallberg A, Bendtzen K, Sener A, Malaisse W J. Endocrinology. 1989;125:752–759. doi: 10.1210/endo-125-2-752. [DOI] [PubMed] [Google Scholar]
- 12.Serreze D V, Gallichan W S, Snider D P, Croitoru K, Rosenthal K L, Leiter E H, Christianson G J, Dudley M E, Roopenian D C. Diabetes. 1996;45:902–908. doi: 10.2337/diab.45.7.902. [DOI] [PubMed] [Google Scholar]
- 13.DiLorenzo T P, Graser R T, Chapman H D, Serreze D V, Ono T, Roopenian D B, Christian G J, Nathenson S G. Proc Natl Acad Sci USA. 1998;95:2538–2543. doi: 10.1073/pnas.95.21.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong F S, Karttunen J, Dumont C, Wen L, Visintin I, Pilip I M, Shastri N, Pamer E G, Janeway C A. Nat Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 15.Graser R T, DiLorenzo T P, Wang F, Christianson G J, Champan H D, Roopenian D C, Nathenson S G, Serreze D V. J Immunol. 2000;164:3913–3918. doi: 10.4049/jimmunol.164.7.3913. [DOI] [PubMed] [Google Scholar]
- 16.Serreze D V, Leiter E H. J Immunol. 1991;147:1222–1229. [PubMed] [Google Scholar]
- 17.Gerling I C, Serreze D V, Christianson S W, Leiter E H. Diabetes. 1992;41:1672–1676. doi: 10.2337/diab.41.12.1672. [DOI] [PubMed] [Google Scholar]
- 18.Rabinovitch A. Diabetes Metab Rev. 1998;14:129–151. doi: 10.1002/(sici)1099-0895(199806)14:2<129::aid-dmr208>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Pugliese A. Diabetes Metab Rev. 1998;14:325–327. doi: 10.1002/(sici)1099-0895(199812)14:4<325::aid-dmr242>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Kubisch H M, Wang J, Bray T M, Phillips J P. Diabetes. 1997;46:1563–1566. doi: 10.2337/diabetes.46.10.1563. [DOI] [PubMed] [Google Scholar]
- 21.Hotta M, Tashiro F, Ikegami H, Niwa H, Ogihara T, Yodoi J, Miyazaki J. J Exp Med. 1998;188:1445–1451. doi: 10.1084/jem.188.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellmann K, Jaattela M, Wissing D, Burkart V, Kolb H. FEBS Lett. 1996;391:185–188. doi: 10.1016/0014-5793(96)00730-2. [DOI] [PubMed] [Google Scholar]
- 23.Janicke R U, Lee F H, Porter A G. Mol Cell Biol. 1994;14:5661–5670. doi: 10.1128/mcb.14.9.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh N, Sun Y, Nakamura K, Smith M R, Colburn N H. Oncol Res. 1995;7:353–362. [PubMed] [Google Scholar]
- 25.Manna S K, Zhang H J, Yan T, Oberley L W, Aggarwal B B. J Biol Chem. 1998;273:13245–13254. doi: 10.1074/jbc.273.21.13245. [DOI] [PubMed] [Google Scholar]
- 26.Bellmann K, Kolb H, Hartmann B, Rothe H, Rowsell P, Rastegar S, Burghardt K, Scott F W. Int J Immunopharmacol. 1997;19:573–577. doi: 10.1016/s0192-0561(97)00052-0. [DOI] [PubMed] [Google Scholar]
- 27.Rabinovitch A, Suarez-Pinzon W, Strynadka K, Ju Q, Edelstein D, Brownlee M, Korbutt G S, Rajotte R V. Diabetes. 1999;48:1223–1229. doi: 10.2337/diabetes.48.6.1223. [DOI] [PubMed] [Google Scholar]
- 28.Eizirik D L, Hoorens A. Mol Cell Biol. 1999;29:47–73. [Google Scholar]