Abstract
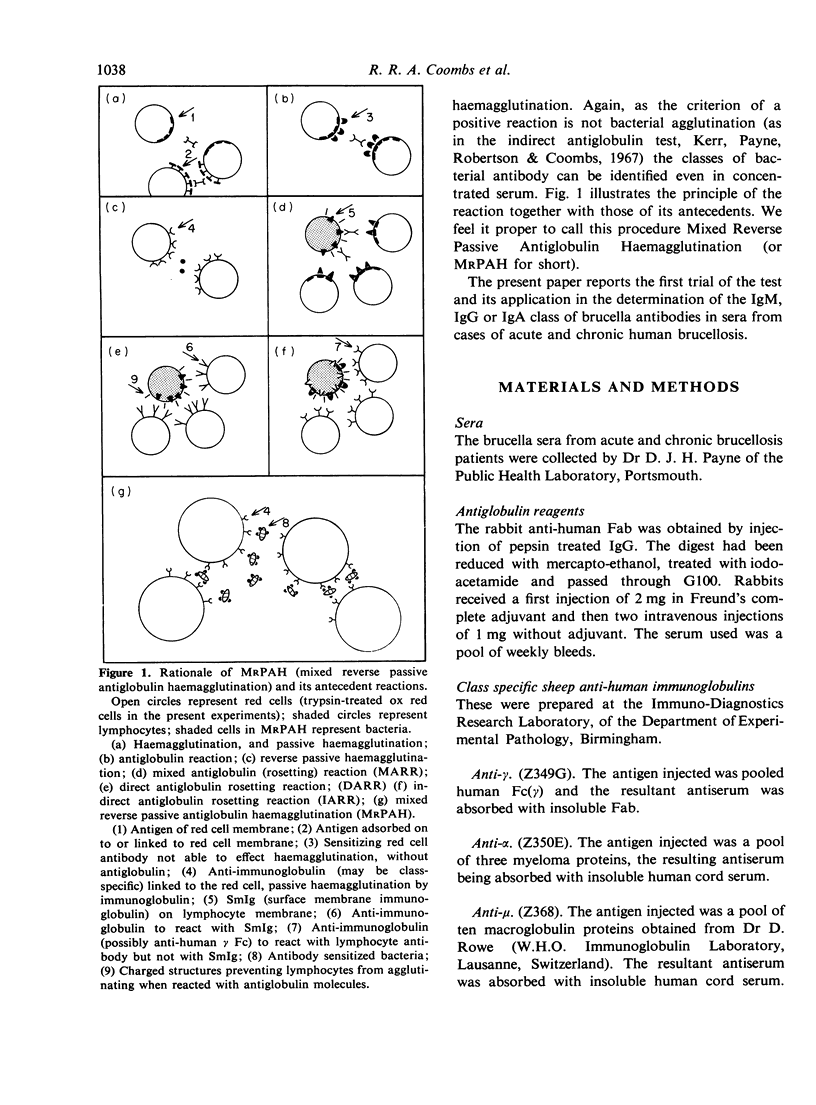
A test is described which is capable of differentiating and measuring by titration the individual classes of antibody reacting with a bacterial suspension. The serum or fluid under test is incubated with the bacteria which are then very well washed and added to indicator red cells linked with specific antiglobulin reagents. Sensitization of the bacteria by a particular class of antibody is shown by haemagglutination (passive) of the appropriate red cells. Agglutination of the bacteria themselves does not preclude an analysis. The reaction, which has been developed on a brucella system, has been designated Mixed Reverse Passive Antiglobulin Haemagglutination (or MRPAH for short).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adinolfi M., Glynn A. A., Lindsay M., Milne C. M. Serological properties of gamma-A antibodies to Escherichia coli present in human colostrum. Immunology. 1966 Jun;10(6):517–526. [PMC free article] [PubMed] [Google Scholar]
- Baublis J. V., Brown G. C. Specific response of the immunoglobulins to rubella infection. Proc Soc Exp Biol Med. 1968 May;128(1):206–210. doi: 10.3181/00379727-128-32979. [DOI] [PubMed] [Google Scholar]
- Bright S., Munro A. J., Lawson Y. A., Joysey V. C., Coombs R. R. An indirect anti-immunoglobulin rosetting reaction to detect alloantibodies to human lymphocytes. J Immunol Methods. 1977;18(1-2):55–62. doi: 10.1016/0022-1759(77)90158-2. [DOI] [PubMed] [Google Scholar]
- Coombs R. R., Wilson A. B., Eremin O., Gurner B. W., Haegert D. G., Lawson Y. A., Bright S., Munro A. J. Comparison of the direct antiglobulin rosetting reaction with the mixed antiglobulin rosetting reaction for the detection of immunoglobulin on lymphocytes. J Immunol Methods. 1977;18(1-2):45–54. doi: 10.1016/0022-1759(77)90157-0. [DOI] [PubMed] [Google Scholar]
- FAGRAEUS A., ESPMARK A. Use of a 'mixed haemadsorption' method in virus-infected tissue cultures. Nature. 1961 Apr 22;190:370–371. doi: 10.1038/190370a0. [DOI] [PubMed] [Google Scholar]
- Feinstein A., Hobart M. J. Structural relationship and complement fixing activity of sheep and other ruminant immunoglobulin G subclasses. Nature. 1969 Aug 30;223(5209):950–952. doi: 10.1038/223950a0. [DOI] [PubMed] [Google Scholar]
- Feinstein A., Munn E. A. Conformation of the free and antigen-bound IgM antibody molecules. Nature. 1969 Dec 27;224(5226):1307–1309. doi: 10.1038/2241307a0. [DOI] [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Kerr W. R., Payne D. J., Robertson L., Coombs R. R. Immunoglobulin class of Brucella antibodies in human sera. Immunology. 1967 Aug;13(2):223–225. [PMC free article] [PubMed] [Google Scholar]
- Thomas V., Shelokov A., Forland M. Antibody-coated bacteria in the urine and the site of urinary-tract infection. N Engl J Med. 1974 Mar 14;290(11):588–590. doi: 10.1056/NEJM197403142901102. [DOI] [PubMed] [Google Scholar]


