Abstract
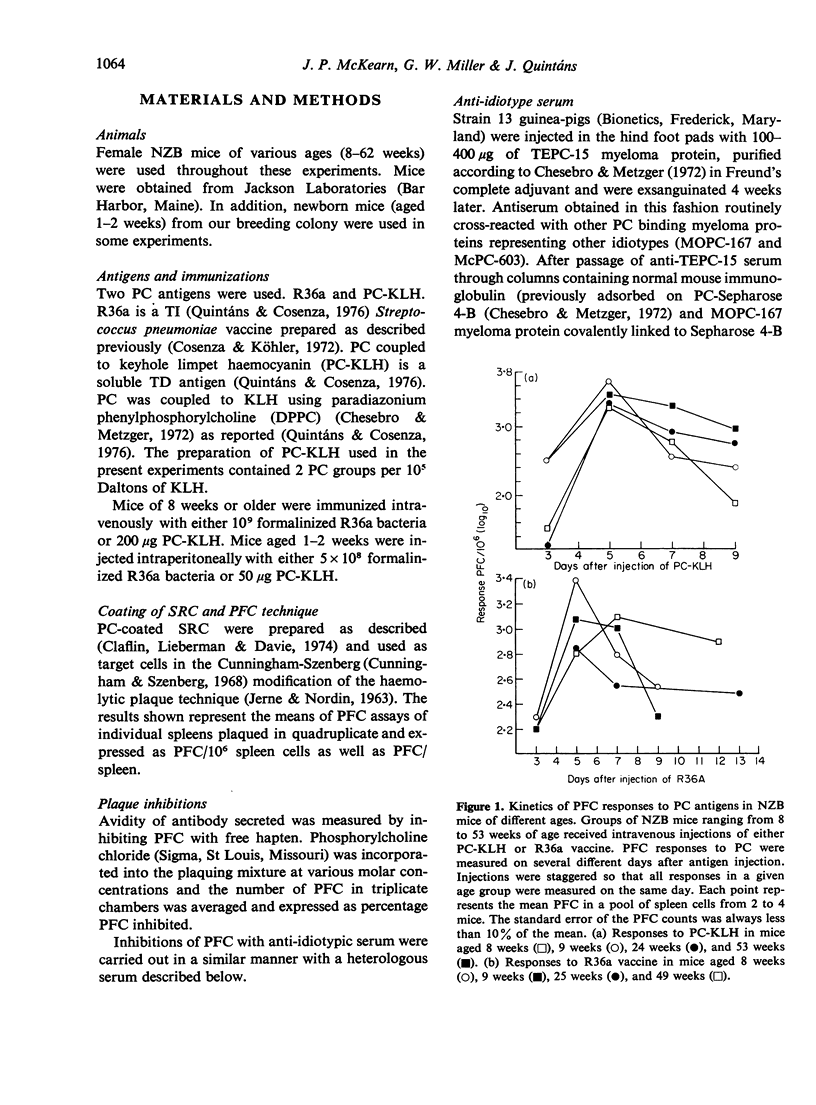
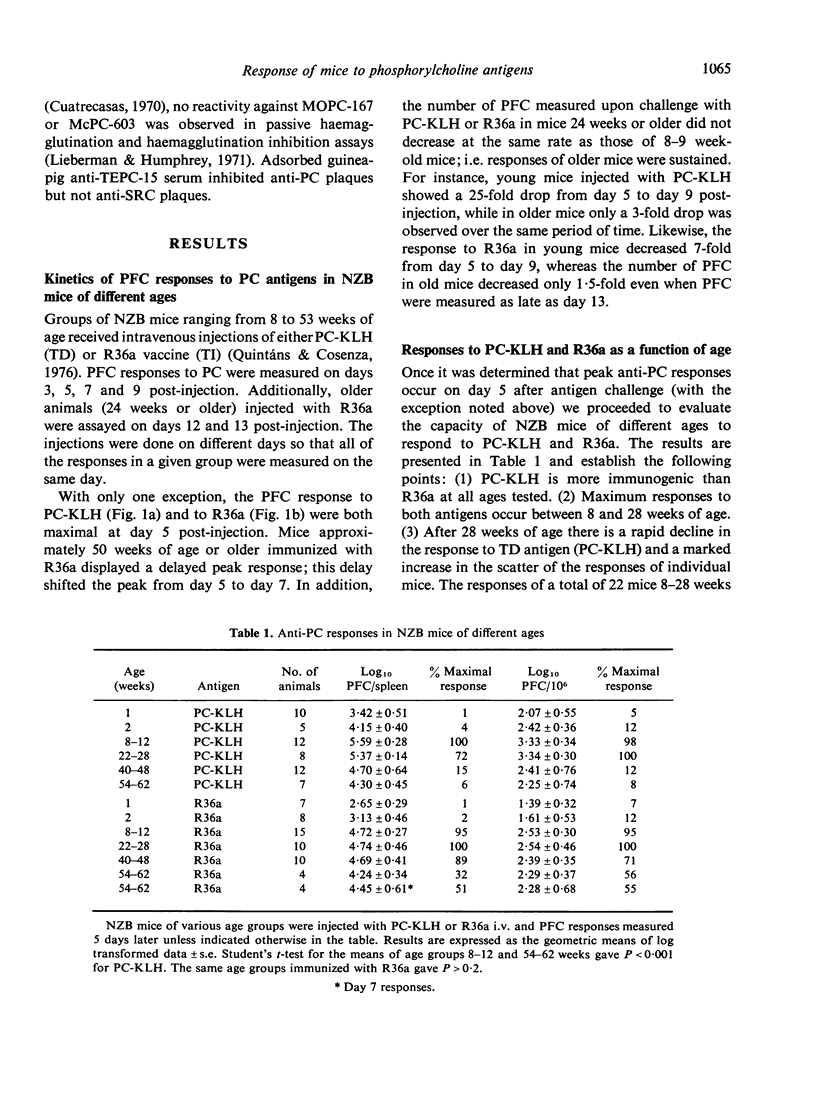
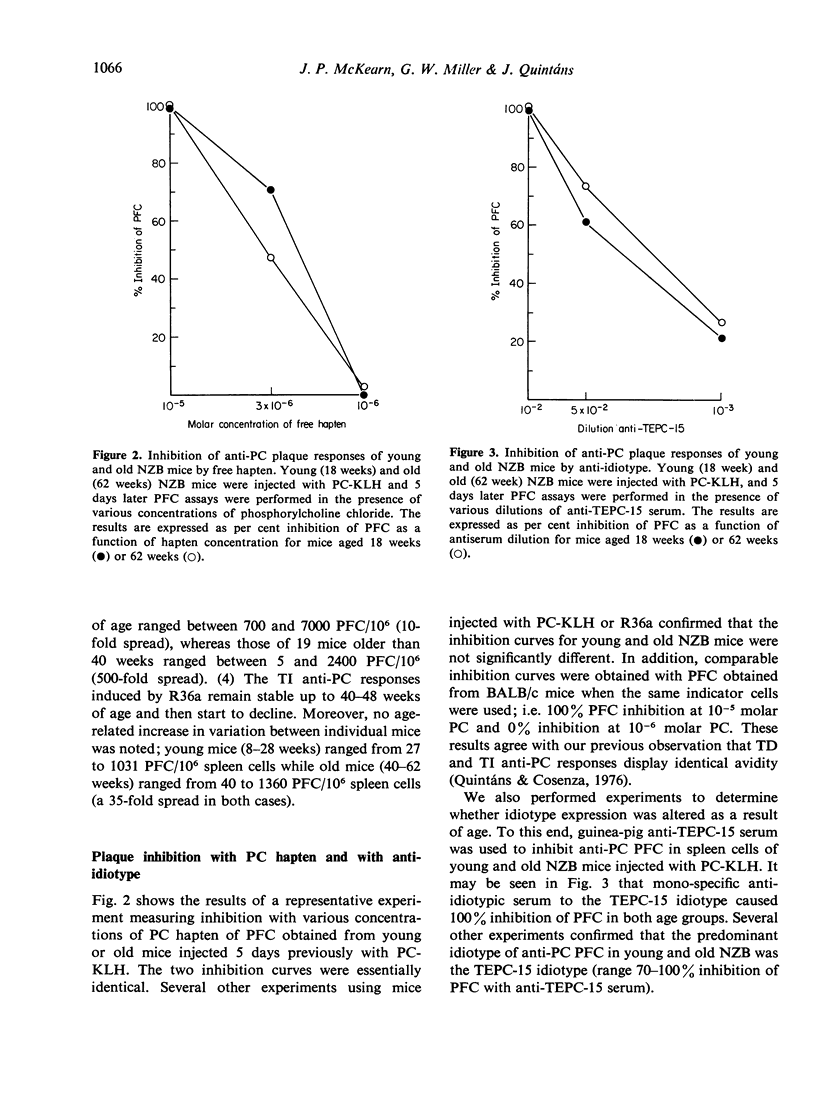
This study was designed to examine aberrations of immune responses in autoimmune NZB strain mice during ageing, at the level of individual B cell clones. The response to phosphorylcholine (PC) was chosen because murine responses to PC are restricted to a few B cell clones and can be characterized with idiotypic markers. Responses to both thymus-dependent (TD) and thymus-independently (TI) PC-containing antigens were measured in mice ranging from 1 to 62 weeks of age. We found that: (1) TD responses to phosphorylcholine keyhole limpet haemocyanin (PC-KLH) decreased markedly (about 17-fold) between 28 and 62 weeks of age. TI responses to the R36a strain pneumococcus decreased only slightly during the same period. (2) The PFC responses to both antigens became markedly prolonged in mice older than 24 weeks. (3) The NZB response to either antigen is essentially monoclonal, as measured by inhibition of PFC with specific anti-idiotype serum and PC hapten. No age-related alteration in avidity or idiotype expression was observed. Our results demonstrate that no aberrant PC-reactive B cell clones appear in old NZB, and lend support to the notion that the abnormalities observed are due to defective regulatory mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Barth R. F., Stashak P. W., Amsbaugh D. F. Enhancement of the antibody response to type 3 pneumococcal polysaccharide in mice treated with antilymphocyte serum. J Immunol. 1970 May;104(5):1313–1315. [PubMed] [Google Scholar]
- Barthold D. R., Kysela S., Steinberg A. D. Decline in suppressor T cell function with age in female NZB mice. J Immunol. 1974 Jan;112(1):9–16. [PubMed] [Google Scholar]
- Baum J. Increased 7S antibody response to sheep erythrocytes in the 2-month-old NZB mouse. Clin Exp Immunol. 1969 Sep;5(3):251–263. [PMC free article] [PubMed] [Google Scholar]
- Blankwater M. J., Levert L. A., Hijmans W. Age-related decline in the antibody response to E. coli lipopolysaccharide in New Zealand Black mice. Immunology. 1975 May;28(5):847–854. [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Lambert P. H., Dixon F. J. Comparison of the immune responsiveness of NZB and NZB X NZW F1 hybrid mice with that of other strains of mice. J Exp Med. 1969 Nov 1;130(5):1093–1105. doi: 10.1084/jem.130.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Claflin J. L., Lieberman R., Davie J. M. Clonal nature of the immune response to phosphorylcholine. I. Specificity, class, and idiotype of phosphorylcholine-binding receptors on lymphoid cells. J Exp Med. 1974 Jan 1;139(1):58–73. doi: 10.1084/jem.139.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin J. L. Uniformity in the clonal repertoire for the immune response to phosphorylcholine in mice. Eur J Immunol. 1976 Oct;6(10):669–674. doi: 10.1002/eji.1830061002. [DOI] [PubMed] [Google Scholar]
- Cosenza H., Köhler H. Specific inhibition of plaque formation to phosphorylcholine by antibody against antibody. Science. 1972 Jun 2;176(4038):1027–1029. doi: 10.1126/science.176.4038.1027. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Immunological maturation. Preferential proliferation of high-affinity precursor cells. J Exp Med. 1973 Jan 1;137(1):201–204. doi: 10.1084/jem.137.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHeer D. H., Edgington T. S. Aberrant maturational characteristics of the immune responses of NZB mice to autologous and heterologous erythrocyte antigens. Cell Immunol. 1975 Oct;19(2):183–193. doi: 10.1016/0008-8749(75)90202-6. [DOI] [PubMed] [Google Scholar]
- Elkerbout E. A., Hijmans W. The long-term antibody response of New Zealand Black mice to sheep red blood cells. Immunology. 1974 May;26(5):893–900. [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. J. Anti-thymocyte serum may enhance or suppress the response to the same antigenic determinant. Immunology. 1975 Nov;29(5):867–874. [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Lieberman R., Humphrey W., Jr Association of H-2 types with genetic control of immune responsiveness to IgA allotypes in the mouse. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2510–2513. doi: 10.1073/pnas.68.10.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor D., Bonavida B., Robinson R. A., Shibata I. N., Percy D. E., Chia D., Barnett E. V. Immune response of New Zealand mice to trinitrophenylated syngeneic mouse red cells. Eur J Immunol. 1976 Nov;6(11):783–789. doi: 10.1002/eji.1830061106. [DOI] [PubMed] [Google Scholar]
- Playfair J. H. Strain differences in the immune responses of mice. 3. A raised tolerance threshold in NZB thymus cells. Immunology. 1971 Dec;21(6):1037–1043. [PMC free article] [PubMed] [Google Scholar]
- Potter M., Lieberman R. Common individual antigenic determinants in five of eight BALB-c IgA myeloma proteins that bind phosphoryl choline. J Exp Med. 1970 Oct 1;132(4):737–751. doi: 10.1084/jem.132.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintáns J., Cosenza H. Antibody response to phosphorylcholine in vitro. II. Analysis of T-dependent and T-independent responses. Eur J Immunol. 1976 Jun;6(6):399–405. doi: 10.1002/eji.1830060605. [DOI] [PubMed] [Google Scholar]
- Sher A., Cohn M. Inheritance of an idiotype associated with the immune response of inbred mice to phosphorylcholine. Eur J Immunol. 1972 Aug;2(4):319–326. doi: 10.1002/eji.1830020405. [DOI] [PubMed] [Google Scholar]
- Staples P. J., Steinberg A. D., Talal N. Induction of immunologic tolerance in older New Zealand mice repopulated with young spleen, bone marrow, or thymus. J Exp Med. 1970 Jun 1;131(6):1223–1238. doi: 10.1084/jem.131.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples P. J., Talal N. Relative inability to induce tolerance in adult NZB and NZB-NZW F1 mice. J Exp Med. 1969 Jan 1;129(1):123–139. doi: 10.1084/jem.129.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann H., Lefkovits I., Quintáns J. Limiting dilution analysis of helper T-cell function. Immunology. 1975 Jun;28(6):1135–1148. [PMC free article] [PubMed] [Google Scholar]
- Weir D. M., McBride W., Naysmith J. D. Immune response to a soluble protein antigen in NZB mice. Nature. 1968 Sep 21;219(5160):1276–1277. doi: 10.1038/2191276a0. [DOI] [PubMed] [Google Scholar]


