Abstract
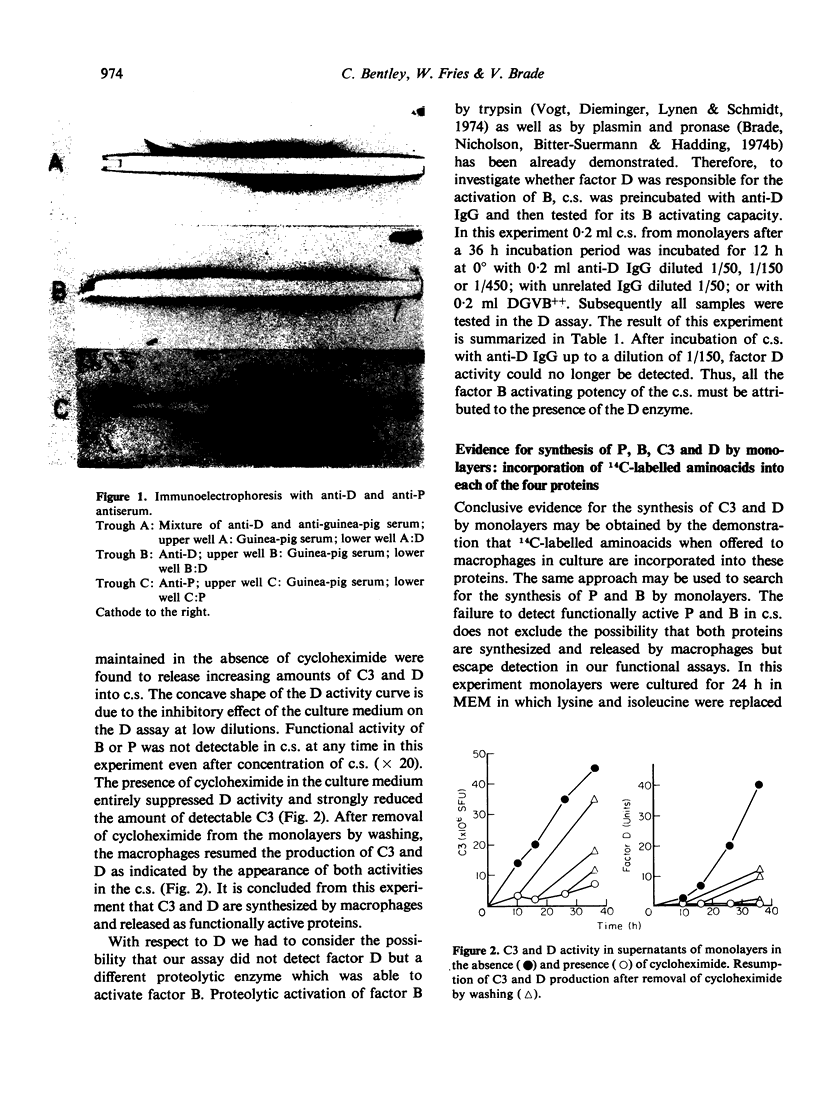
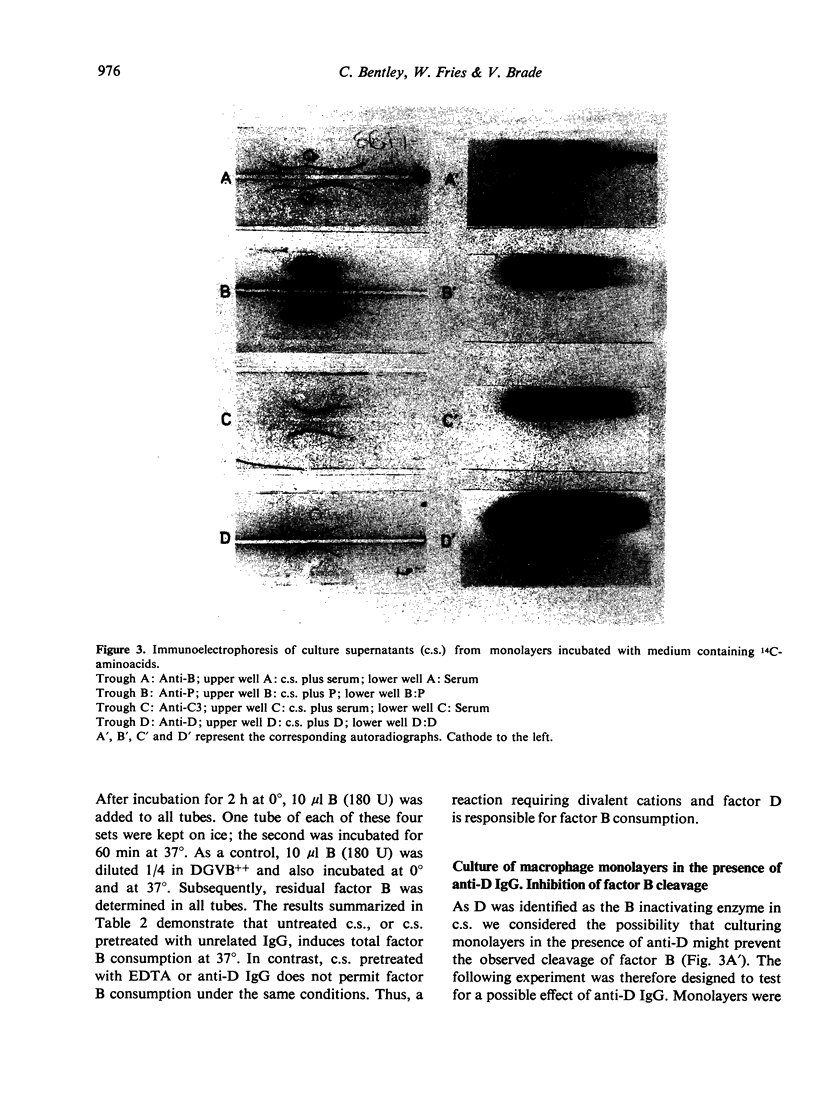
Culture supernatants from monolayers prepared with guinea-pig peritoneal macrophages were found to contain functional D and C3 activity. Factor D was detected by consumption of C3 in the presence of culture supernatant, factor B and insoluble C3b. Preincubation of culture supernatant with anti-D IgG totally inhibited C3 consumption in the D assay which identified factor D as the B activating enzyme. The synthesis of D and C3 by macrophages was proven by the fact that cycloheximide in the culture medium strongly reduced the amount of detectable D and C3 and also incorporation experiments with 14C-labelled aminoacids resulted in the production by macrophages of radio-labelled D and C3. In addition radiolabelled B and P were also detected. The majority of the B protein appeared in its cleaved (Bb) form and, therefore, factor B escaped detection in the functional assay. For unknown reasons functional activity of P was not detectable. The enzyme responsible for B cleavage in the culture supernatants was identified as factor D. Activation of B by the D enzyme in culture supernatants probably occurred through the C3b-dependent feed back cycle of the alternative pathway. This is concluded from our observation that C3 was consumed on incubation of the culture supernatant at 37°, although at a low rate.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Johnson A. M., Birtch A. G., Moore F. D. Human C'3: evidence for the liver as the primary site of synthesis. Science. 1969 Jan 17;163(3864):286–288. doi: 10.1126/science.163.3864.286. [DOI] [PubMed] [Google Scholar]
- Bentley C., Bitter-Suermann D., Hadding U., Brade V. In vitro synthesis of factor B of the alternative pathway of complement activation by mouse peritoneal macrophages. Eur J Immunol. 1976 Jun;6(6):393–398. doi: 10.1002/eji.1830060604. [DOI] [PubMed] [Google Scholar]
- Bentley C., Hadding U., Bitter-Suermann D., Brade V. Effect of in vivo stimulation of mice on the secretion of factor B of the alternate complement pathway by peritoneal macrophages. Eur J Immunol. 1977 Mar;7(3):188–190. doi: 10.1002/eji.1830070315. [DOI] [PubMed] [Google Scholar]
- Bitter-Suermann D., Hadding U., Melchert F., Wellensiek H. J. Independent and consecutive action of the complement components C5, C6 and C7 in immune hemolysis. I. Preparation of EAC1-5 with purified guinea pig C3 and C5. Immunochemistry. 1970 Dec;7(12):955–965. doi: 10.1016/0019-2791(70)90002-9. [DOI] [PubMed] [Google Scholar]
- Brade V., Bentley C., Bitter-Suermann D., Hadding U. Interaction of zymosan and of activated properdin with factor D-depleted guinea pig serum: implications for the mechanism of initial C3 cleavage via the alternative complement pathway. Z Immunitatsforsch Immunobiol. 1977 Feb;152(5):402–414. [PubMed] [Google Scholar]
- Brade V., Cook C. T., Shin H. S., Mayer M. M. Studies on the properdin system: isolation of a heat-labile factor from guinea pig serum related to a human glycine rich beta-glycoprotein (GBG or factor B). J Immunol. 1972 Dec;109(6):1174–1181. [PubMed] [Google Scholar]
- Brade V., Dieminger L., Schmidt G., Vogt W. Incompatibility between C3b and B of guinea-pig and man and its influence on the titration of the alternative pathway factors D and B in these two species. Immunology. 1976 Feb;30(2):171–179. [PMC free article] [PubMed] [Google Scholar]
- Brade V., Hall R. E., Colten H. R. Biosynthesis of pro-C3, a precursor of the third component of complement. J Exp Med. 1977 Sep 1;146(3):759–765. doi: 10.1084/jem.146.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade V., Nicholson A., Bitter-Suermann D., Hadding U. Formation of the C3-cleaving properdin enzyme on zymosan. Demonstration that factor D is replaceable by proteolytic enzymes. J Immunol. 1974 Dec;113(6):1735–1743. [PubMed] [Google Scholar]
- Brade V., Nicholson A., Lee G. D., Mayer M. M. The reaction of zymosan with the properdin system: isolation of purified factor D from guinea pig serum and study of its reaction characteristics. J Immunol. 1974 May;112(5):1845–1854. [PubMed] [Google Scholar]
- Colten H. R. Biosynthesis of complement. Adv Immunol. 1976;22:67–118. doi: 10.1016/s0065-2776(08)60548-9. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Properdin factor D: characterization of its active site and isolation of the precursor form. J Exp Med. 1974 Feb 1;139(2):355–366. doi: 10.1084/jem.139.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Macrophage neutral proteinases and defense of the lung. Fed Proc. 1977 Dec;36(13):2707–2711. [PubMed] [Google Scholar]
- McClelland D. B., Furth R. V. In vitro synthesis of beta1C/beta1A globulin (the C3 component of complement) by tissues and leucocytes of mice. Immunology. 1976 Dec;31(6):855–861. [PMC free article] [PubMed] [Google Scholar]
- Nicholson A., Brade V., Schorlemmer H. U., Burger R., Bitter-Suermann D., Hadding U. Interaction of C3b, B, and D in the alternative pathway of complement activation. J Immunol. 1975 Oct;115(4):1108–1113. [PubMed] [Google Scholar]
- Schorlemmer H. U., Allison A. C. Effects of activated complement components on enzyme secretion by macrophages. Immunology. 1976 Nov;31(5):781–788. [PMC free article] [PubMed] [Google Scholar]
- Stecher V. J., Morse J. H., Thorbecke G. J. Sites of production of primate serum proteins associated with complement system. Proc Soc Exp Biol Med. 1967 Feb;124(2):433–438. doi: 10.3181/00379727-124-31758. [DOI] [PubMed] [Google Scholar]
- Vogt W., Dieminger L., Lynen R., Schmidt G. Alternative pathway for the activation of complement in human serum. Formation and composition of the complex with cobra venom factor that cleaves the third component of complement. Hoppe Seylers Z Physiol Chem. 1974 Feb;355(2):171–183. doi: 10.1515/bchm2.1974.355.1.171. [DOI] [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by endotoxin-activated macrophages. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3598–3601. doi: 10.1073/pnas.71.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]





