Abstract
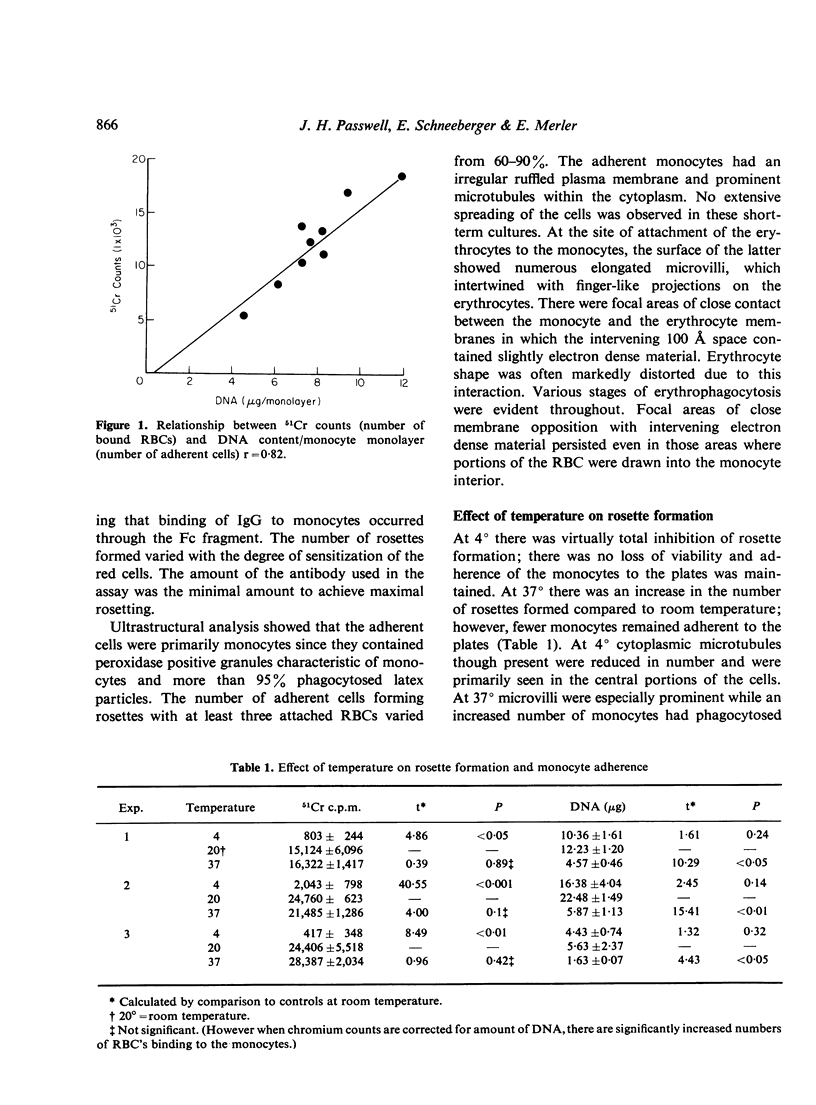
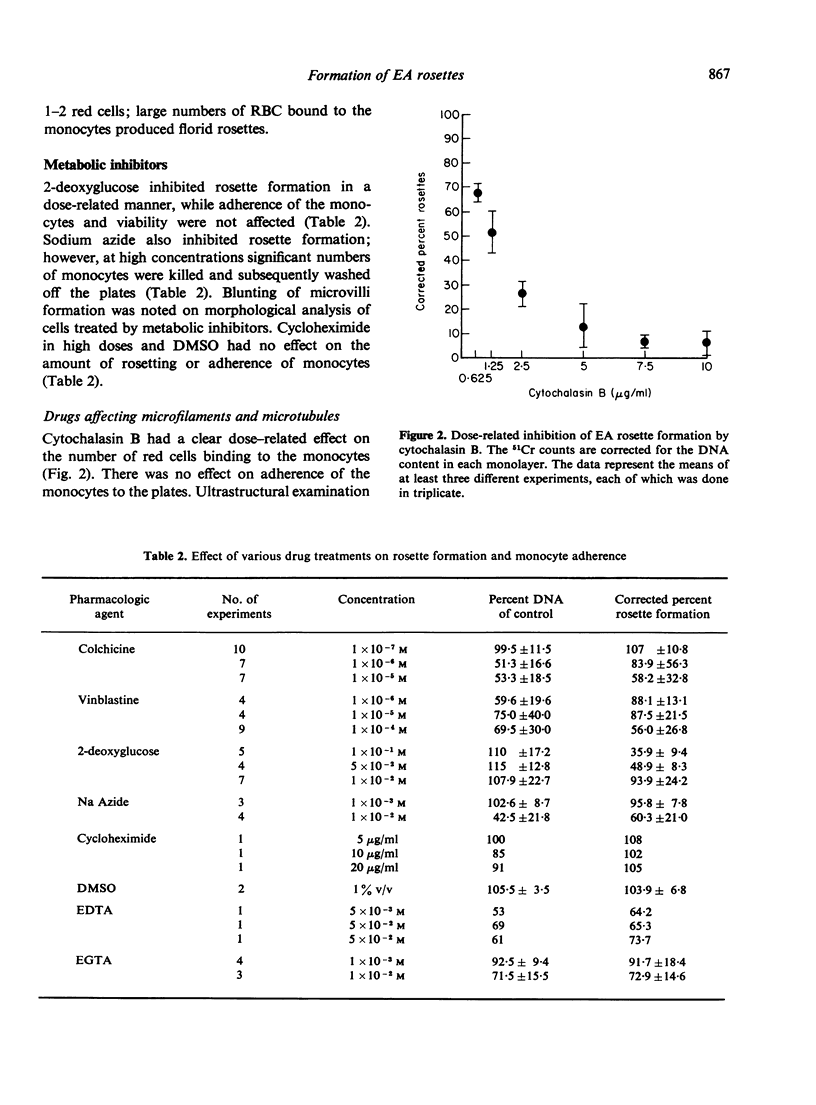
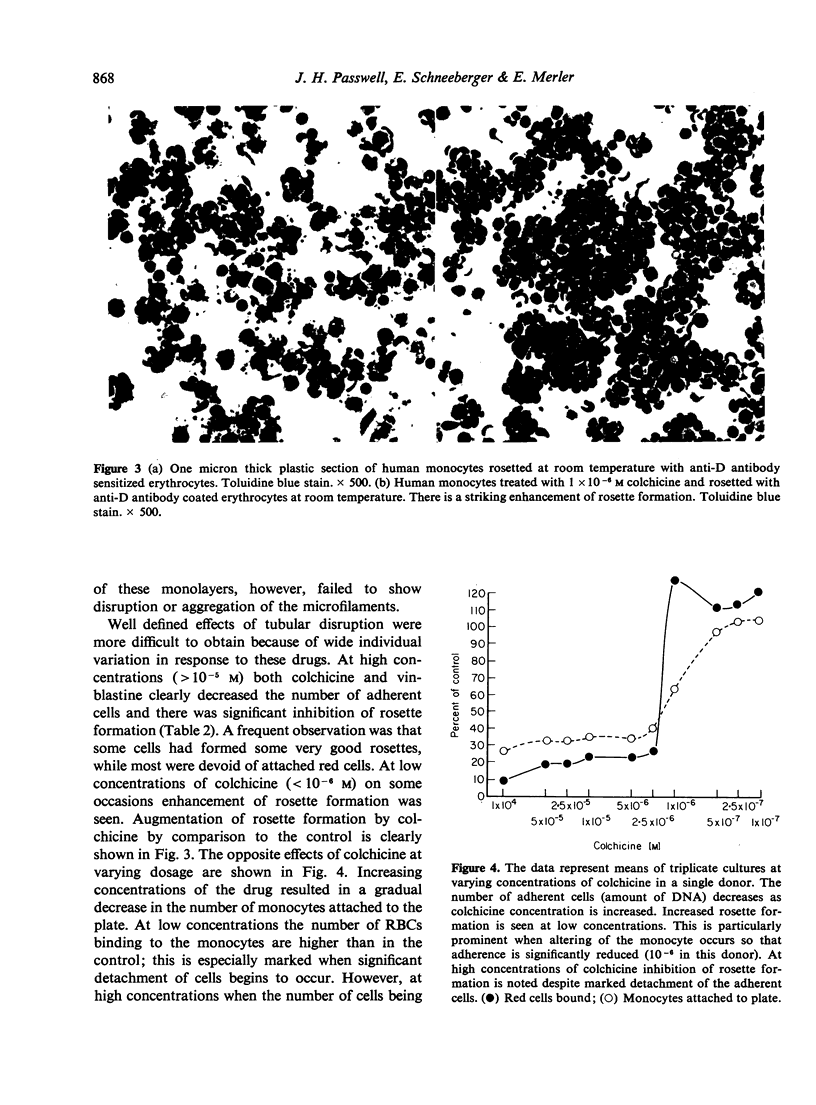
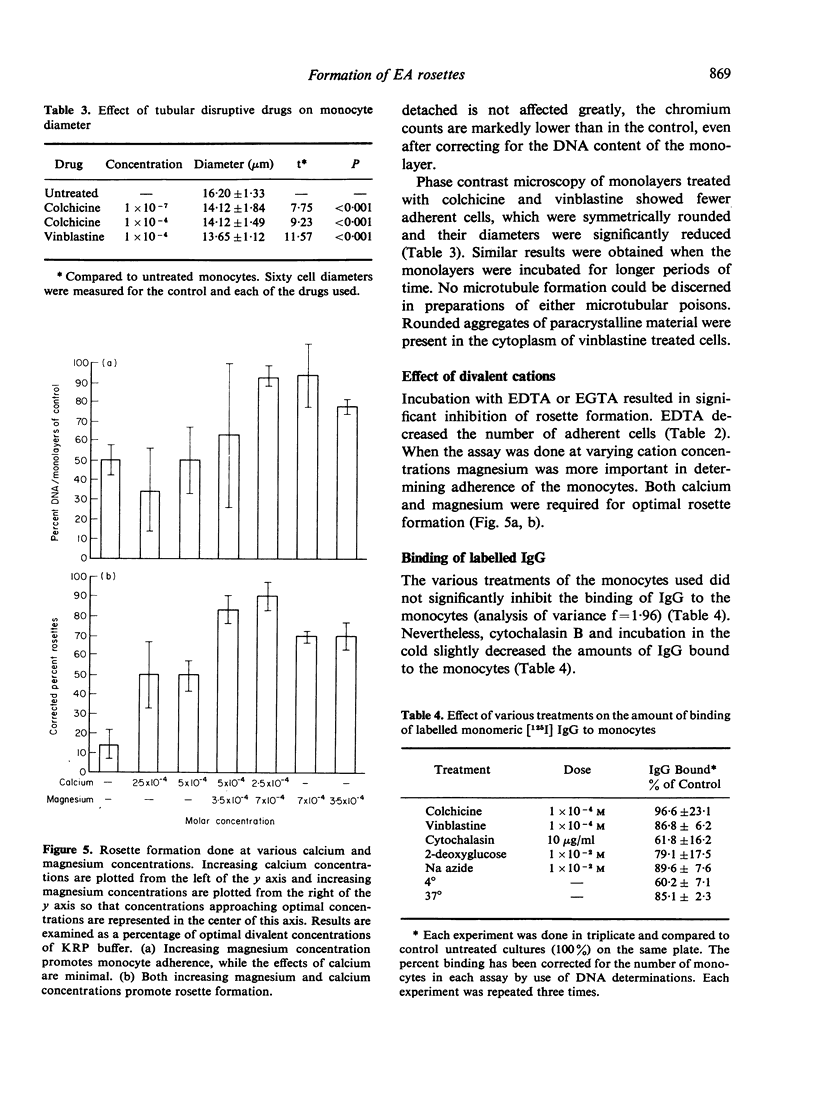
The binding of sensitized red cells to Fc receptors in human monocytes was studied by evaluating the effects of various pharmacological reagents and other treatments on EA rosette formation. Cytochalasin B and 2-deoxyglucose inhibited rosette formation in a dose-dependent manner. Sodium azide and incubation at 4 degrees also inhibited rosette formation, while at 37 degrees increased numbers of RBCs bound to the monocytes. The microtubular poisons, vinblastine and colchicine at high concentrations resulted in decreased adherence of monocytes and inhibition of rosette formation, while at low concentrations of colchicine, enhanced rosette formation was sometimes observed. Contrary to the effects on rosette formation, binding of [125I] IgG to monocyte monolayers was not altered by treatment of the monocytes with drugs. Magnesium ions were required to promote monocyte adherence, but both magnesium and calcium were needed for the best rosette formation. We conclude that the formation of EA rosettes is dependent not merely on binding of IgG to the Fc receptor but requires metabolically active monocytes, an intact cytostructure and suitable environmental conditions (temperature and cation concentration).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson N., Gelfand E. W., Jandl J. H., Rosen F. S. The interaction between human monocytes and red cells. Specificity for IgG subclasses and IgG fragments. J Exp Med. 1970 Dec 1;132(6):1207–1215. doi: 10.1084/jem.132.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson N., Lo Buglio A. F., Jandl J. H., Cotran R. S. The interaction between human monocytes and red cells. Binding characteristics. J Exp Med. 1970 Dec 1;132(6):1191–1206. doi: 10.1084/jem.132.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. P., Michael J. M., Chaplin H., Jr, Parker C. W. Modulation of macrophage C3b receptor function by cytochalasin-sensitive structures. J Immunol. 1977 Apr;118(4):1292–1299. [PubMed] [Google Scholar]
- Atkinson J. P., Parker C. W. Modulation of macrophage Fc receptor function by cytochalasin-sensitive structures. Cell Immunol. 1977 Oct;33(2):353–363. doi: 10.1016/0008-8749(77)90164-2. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Davis A. T., Estensen R. D., Quie P. G. Cytochalasin B. IV. Inhibition and stimulation of chemotaxis of rabbit and human polymorphonuclear leukocytes. J Immunol. 1972 Feb;108(2):396–402. [PubMed] [Google Scholar]
- Ciccimarra F., Rosen F. S., Merler E. Localization of the IgG effector site for monocyte receptors. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2081–2083. doi: 10.1073/pnas.72.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN E. C. Structural units of human 7S gamma globulin. J Clin Invest. 1960 Dec;39:1933–1941. doi: 10.1172/JCI104218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand E. W., Morris S. A., Resch K. Antibody-dependent cytotoxicity: modulation by the cytochalasins and microtubule-disruptive agents. J Immunol. 1975 Mar;114(3):919–924. [PubMed] [Google Scholar]
- Herskovitz B., Guerry D., 4th, Cooper R. A., Schreiber A. D. Effect of cytochalasin B on human monocyte binding and sphering of IgG-coated human erythrocytes. Blood. 1977 Feb;49(2):289–294. [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBuglio A. F., Cotran R. S., Jandl J. H. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967 Dec 22;158(3808):1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- Mayhew E., Maslow D. E. Cytochalasin B and the sialic acids of Ehrlich ascites cells. Exp Cell Res. 1974 Feb;83(2):255–260. doi: 10.1016/0014-4827(74)90337-1. [DOI] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. I. Description of the inhibitory effect. J Exp Med. 1976 Dec 1;144(6):1465–1483. doi: 10.1084/jem.144.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor G. O., Turner M. W., Hay F. C. Localisation of monocyte binding site of human immunoglobulin G. Nature. 1974 Mar 15;248(445):228–230. doi: 10.1038/248228a0. [DOI] [PubMed] [Google Scholar]
- Pesanti E. L., Axline S. G. Colchicine effects on lysosomal enzyme induction and intracellular degradation in the cultivated macrophage. J Exp Med. 1975 May 1;141(5):1030–1046. doi: 10.1084/jem.141.5.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. Macrophage heterogeneity in receptor activity: the activation of macrophage Fc receptor function in vivo and in vitro. J Immunol. 1975 Mar;114(3):976–981. [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D. Disappearance and recovery of human monocyte IgG receptor activity after phagocytosis. J Immunol. 1972 Oct;109(4):914–917. [PubMed] [Google Scholar]
- Schreiber A. D., Frank M. M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Invest. 1972 Mar;51(3):575–582. doi: 10.1172/JCI106846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Ukena T. E., Berlin R. D. Effect of colchicine and vinblastine on the topographical separation of membrane functions. J Exp Med. 1972 Jul 1;136(1):1–7. doi: 10.1084/jem.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Karnovsky M. J., Engers H. D. Ligand-induced movement of lymphocyte membrane macromolecules. 3. Relationship between the formation and fate of anti-Ig-surface Ig complexes and cell metabolism. J Exp Med. 1973 Mar 1;137(3):675–689. doi: 10.1084/jem.137.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Yoshinaga M., Yoshinaga A., Waksman B. H. Regulation of lymphocyte responses in vitro: potentiation and inhibition of rat lymphocyte responses to antigen and mitogens by cytochalasin B. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3251–3255. doi: 10.1073/pnas.69.11.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]



