Abstract
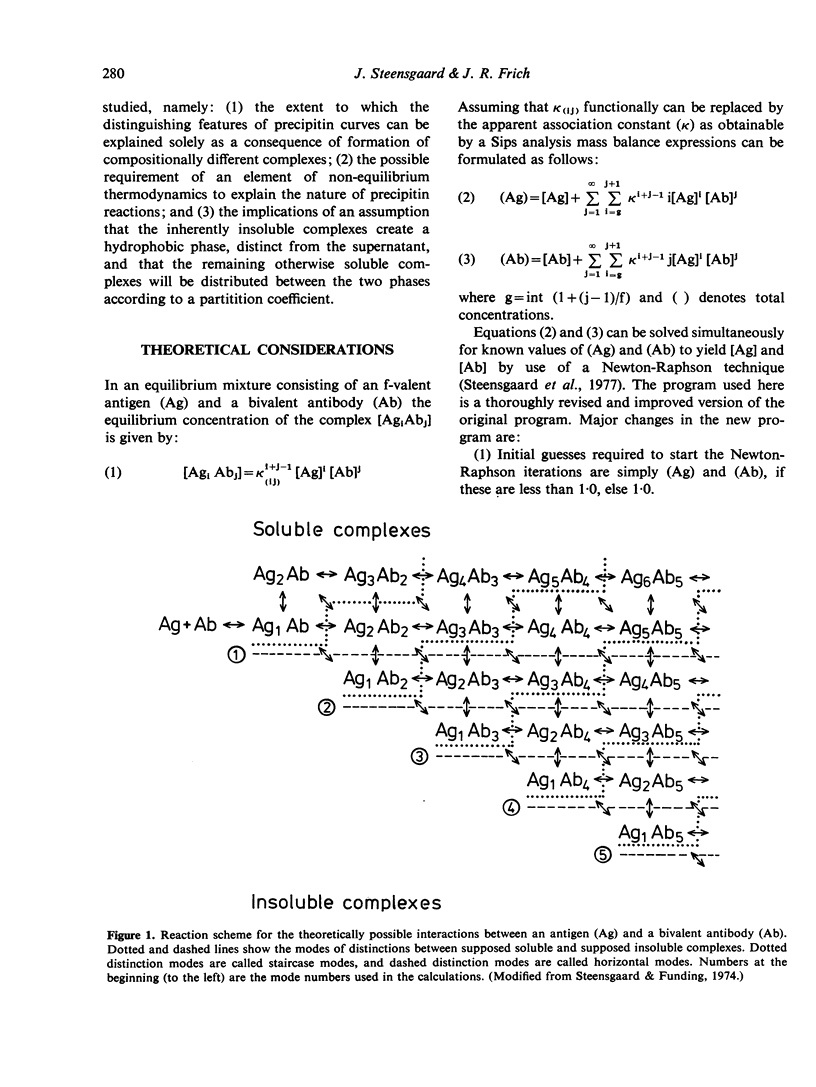
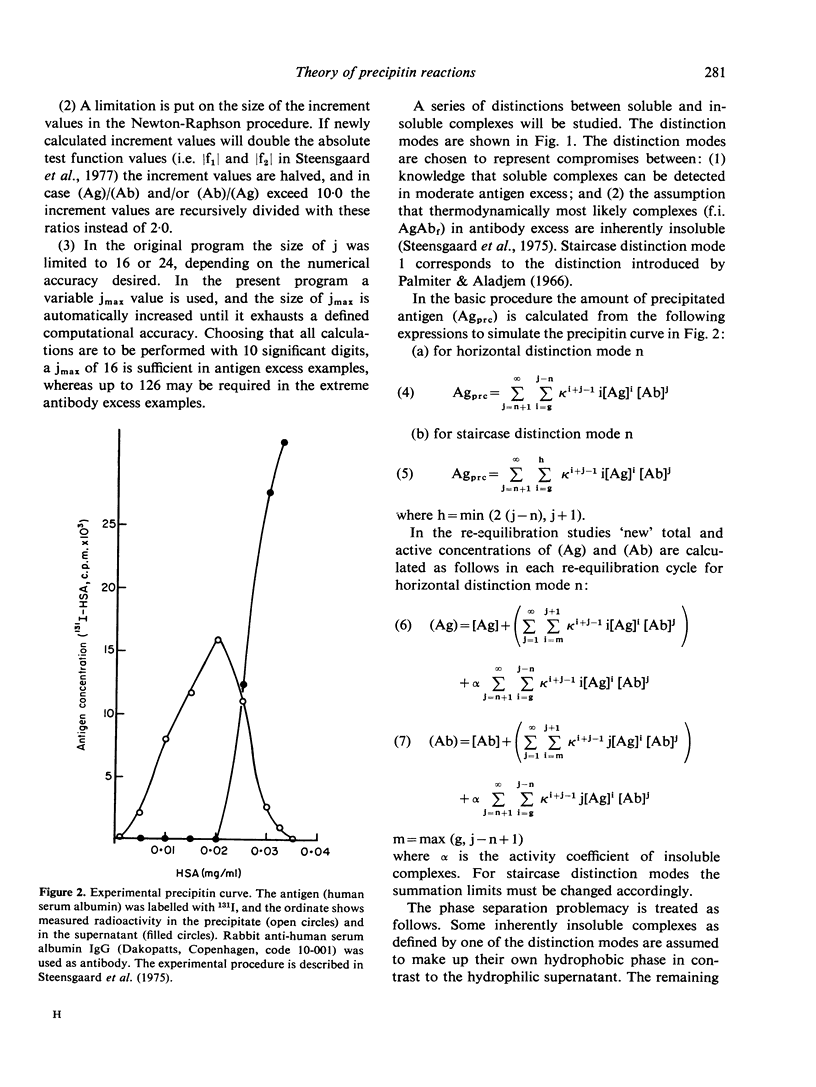
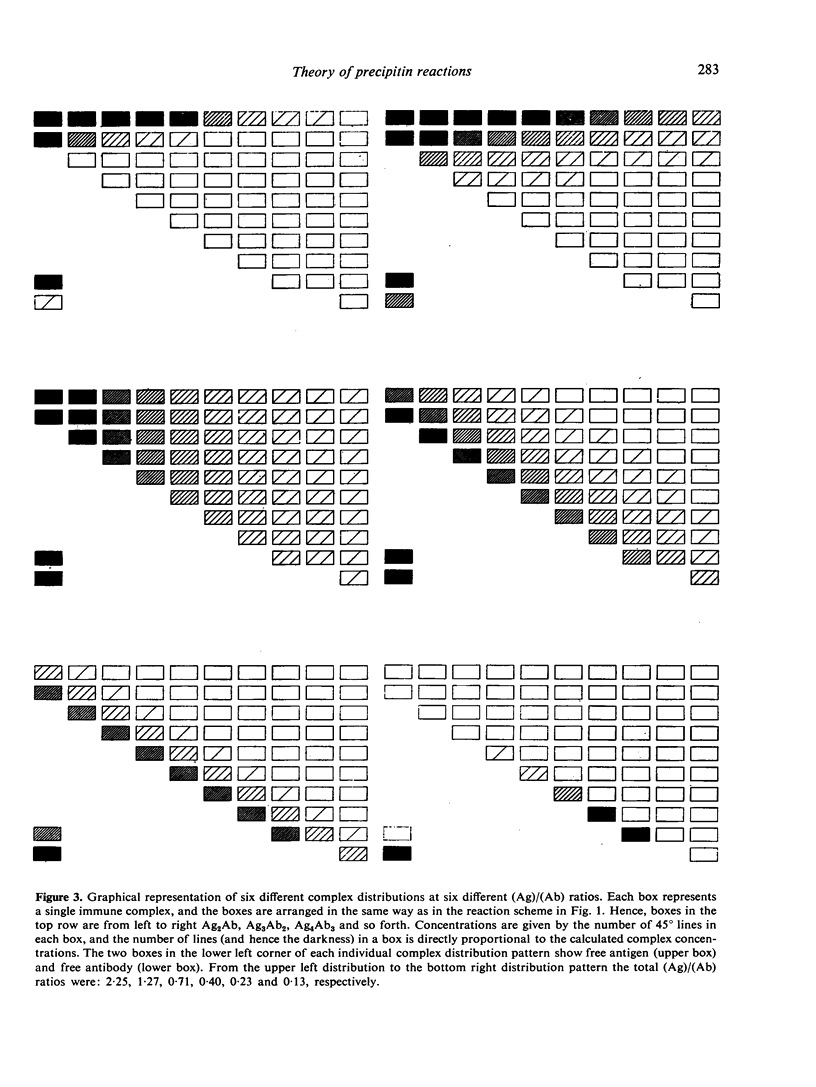
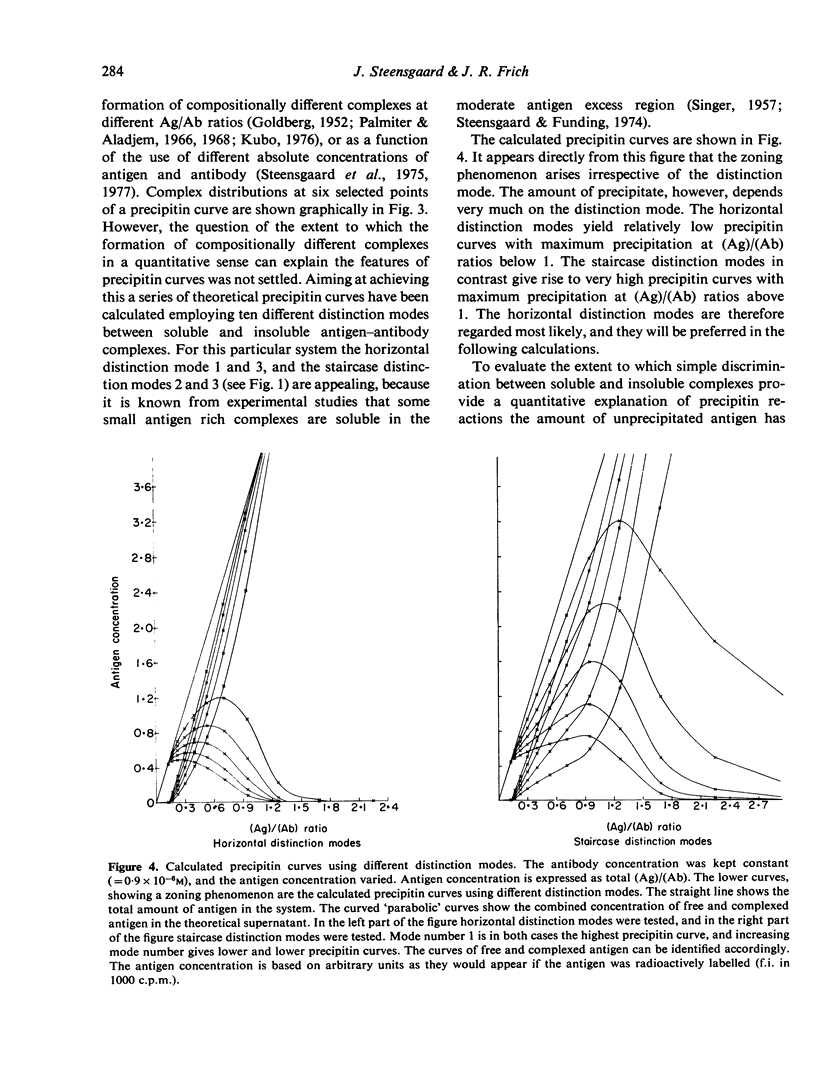
The theoretical consequences of different hypotheses of the mechanism of precipitin reactions have been evaluated by means of computer simulation. It has been found that the formation of compositionally different complexes in different antigen/antibody mixtures provides a valid explanation of the zoning phenomenon, but this concept fails to explain the absence of free antigen and of antigen in soluble complexes at the point of maximum percipitation. It is found that the following hypothesis provides an improved qualitative and quantitative explanation of percipitin reactions. In the first stage of the total reaction a series of compositionally different complexes is formed. As the second stage of the total reaction two kinds of processes are proposed. Inherently insoluble complexes precipitate causing the remaining soluble complexes to participate in mutual rearrangements to re-establish a new state of equilibrium in the supernatant. The inherently insoluble complexes, moreover, create a hydrophobic phase, distinct from the supernatant and cause the remaining otherwise soluble complexes to distribute themselves between the two phases according to a partition coefficient. A mathematical apparatus to study the consequences of this hypothesis is presented, and it is demonstrated that the features of precipitin curves can be explained nearly completely this way.
Full text
PDF

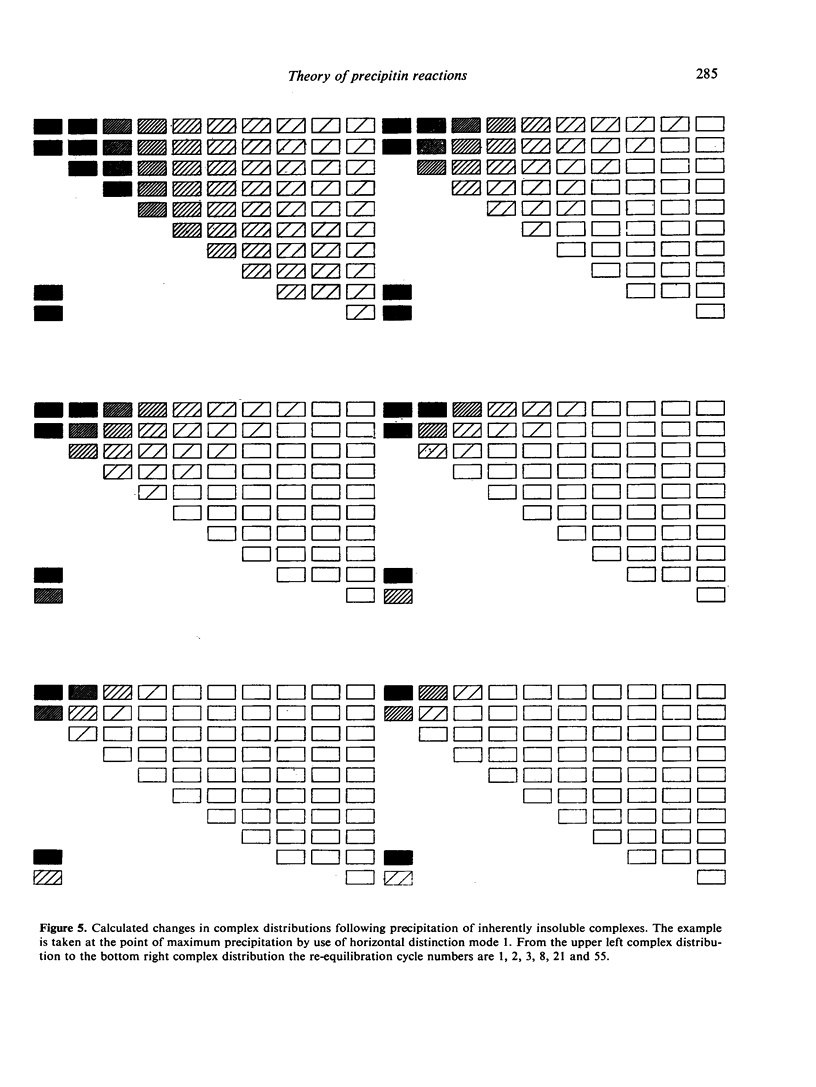
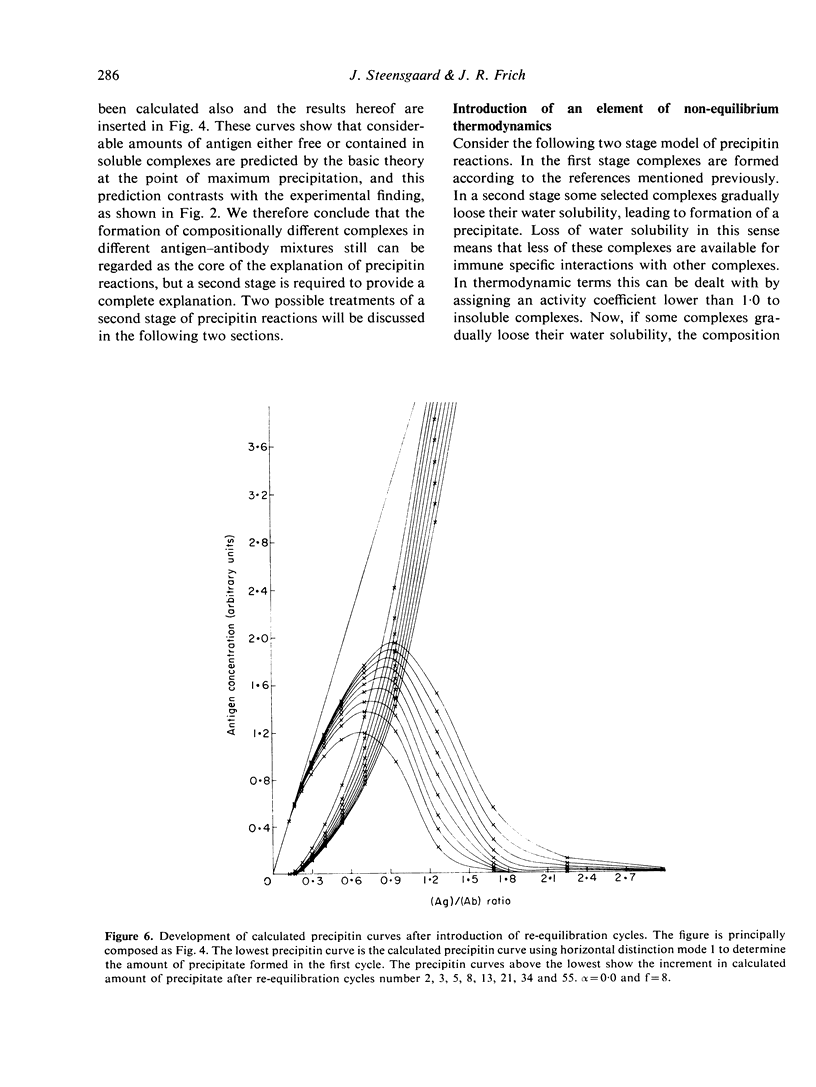
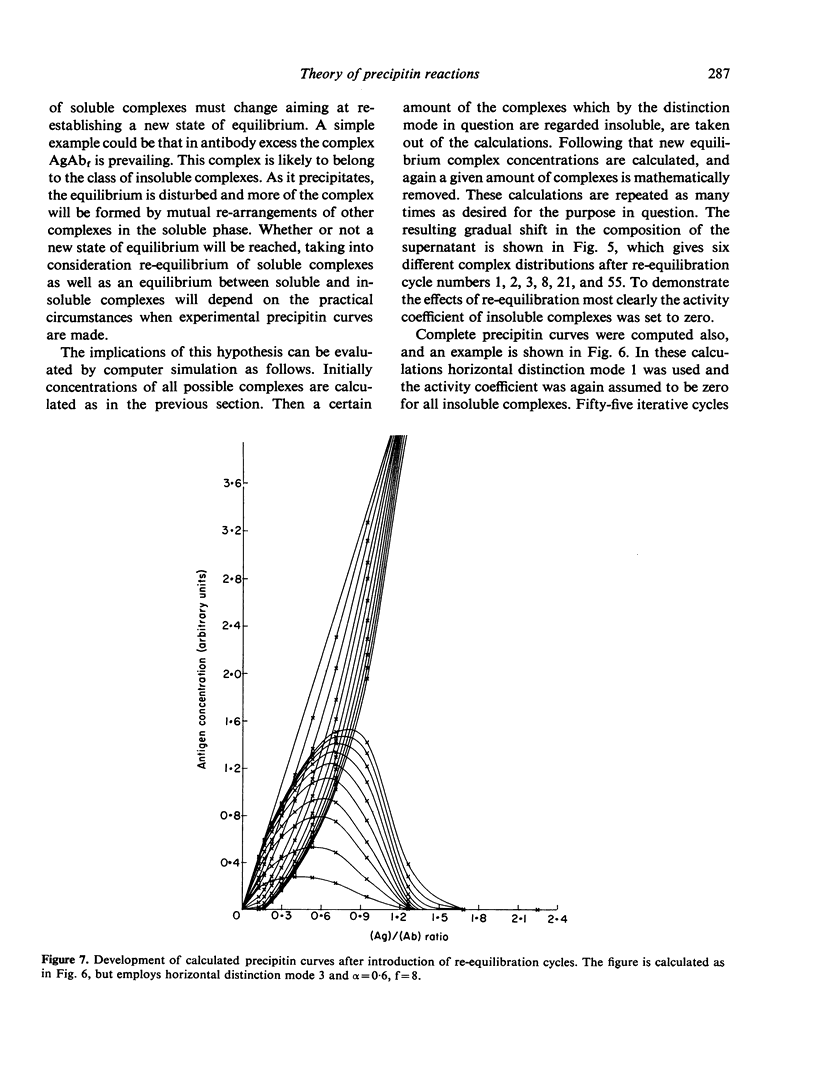
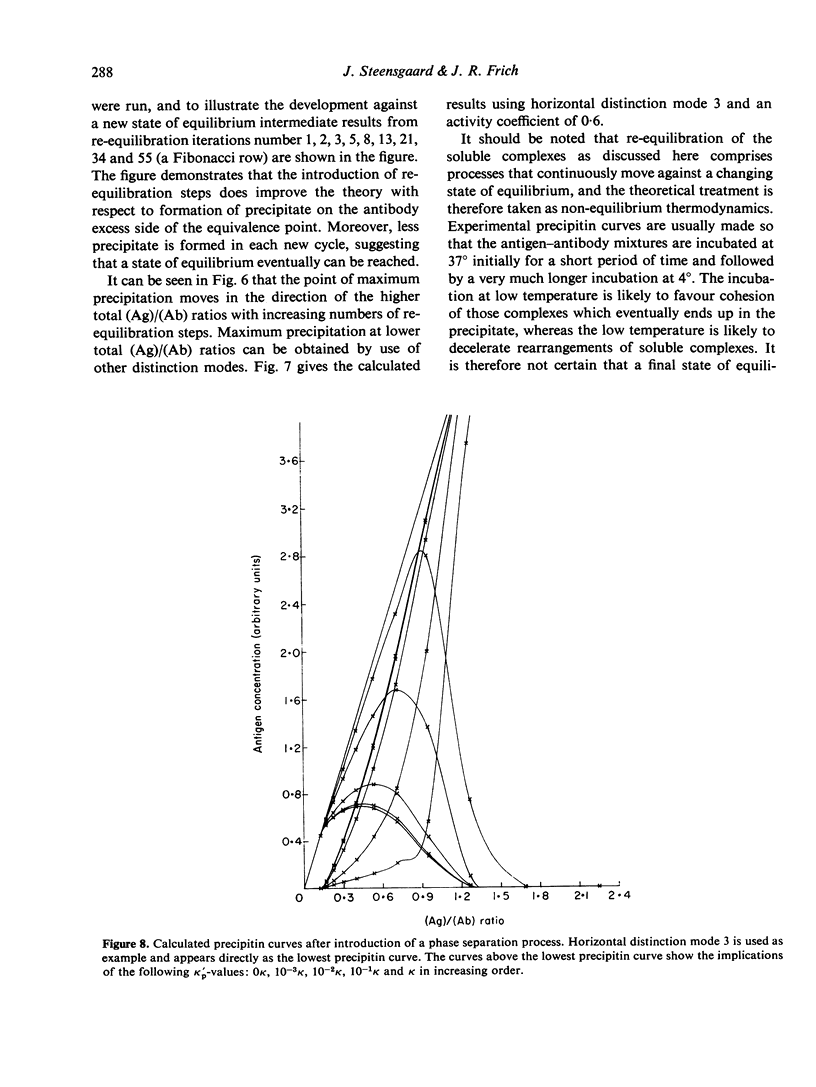


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Mannik M. Determination of soluble immune complex molar composition and antibody association constants by ammonium sulfate precipitation. J Immunol. 1974 Feb;112(2):451–461. [PubMed] [Google Scholar]
- Klubo K. A view on the equivalence point in the precipitin test. J Theor Biol. 1976 Dec;63(2):453–478. doi: 10.1016/0022-5193(76)90046-1. [DOI] [PubMed] [Google Scholar]
- Palmiter M. T., Aladjem F. On the composition of insoluble antigen-antibody complexes. J Theor Biol. 1968 Jan;18(1):34–52. doi: 10.1016/0022-5193(68)90169-0. [DOI] [PubMed] [Google Scholar]
- Palmiter M. T., Aladjem F. The antigen-antibody reaction. IV. A quantitative theory of antigen-antibody reactions. J Theor Biol. 1963 Sep;5(2):211–235. doi: 10.1016/0022-5193(63)90060-2. [DOI] [PubMed] [Google Scholar]
- Steensgaard J., Funding L. On the formulation of a reaction scheme for the interaction between an antigen and its antibody. Immunology. 1974 Feb;26(2):299–302. [PMC free article] [PubMed] [Google Scholar]
- Steensgaard J., Johansen H. K., Moller N. P. Computer simulation of immunochemical interactions. Immunology. 1975 Sep;29(3):571–579. [PMC free article] [PubMed] [Google Scholar]
- Steensgaard J., Liu B. M., Cline G. B., Møller N. P. The properties of immune complex-forming systems. A new theoretical approach. Immunology. 1977 Apr;32(4):445–456. [PMC free article] [PubMed] [Google Scholar]


