Abstract
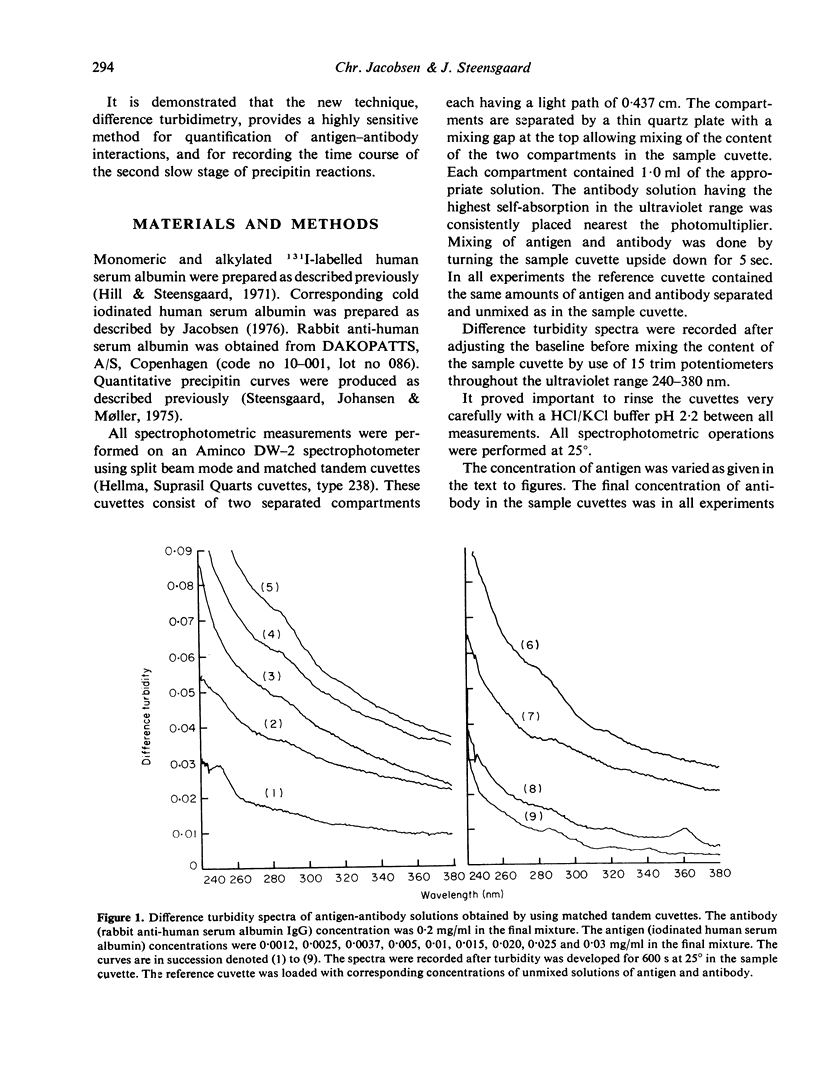
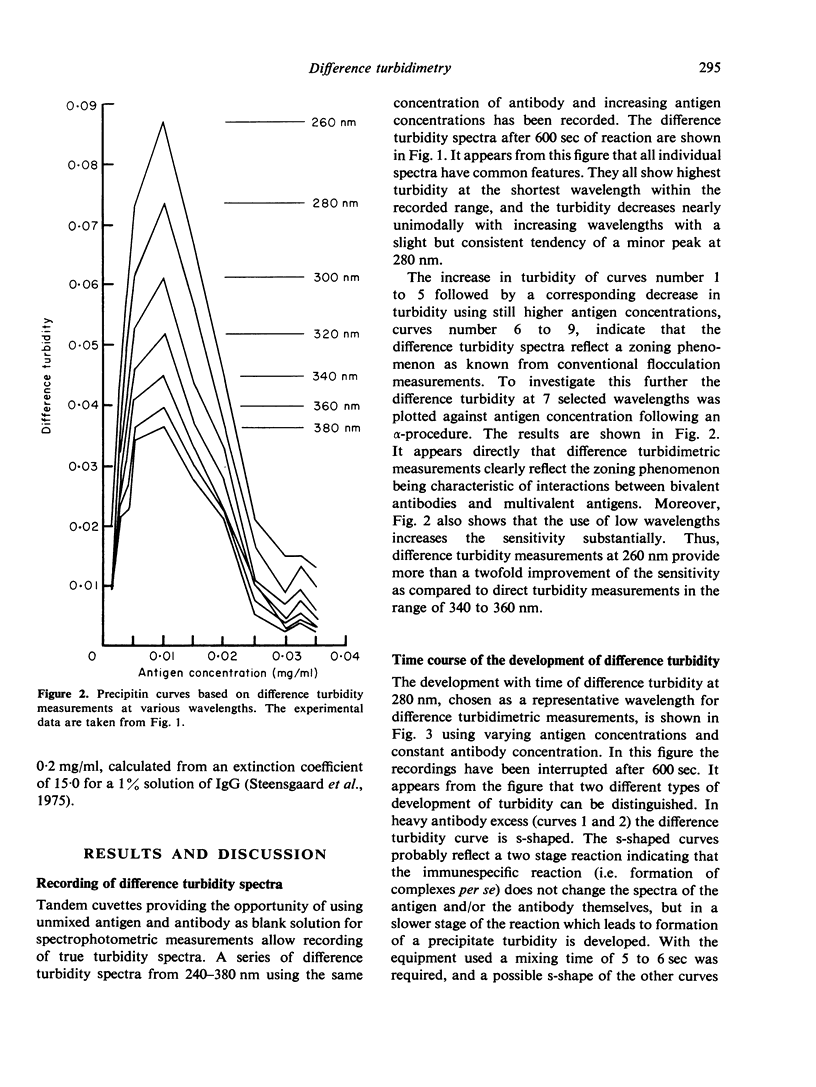
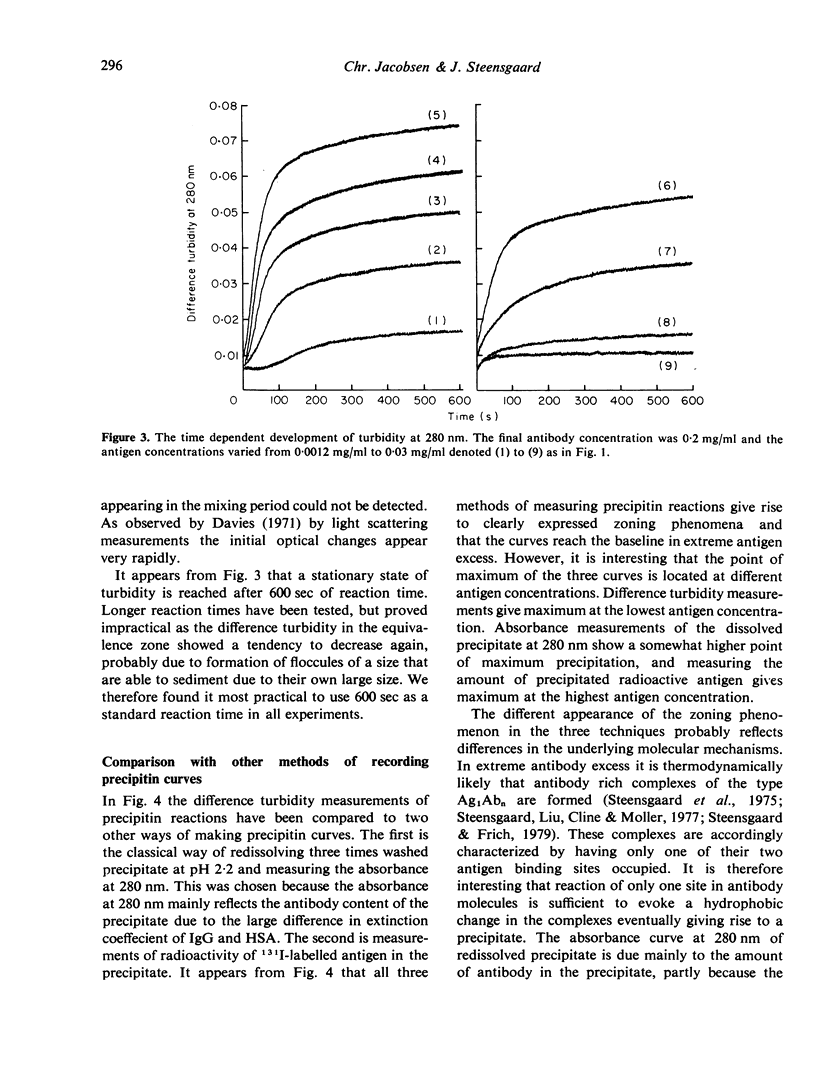
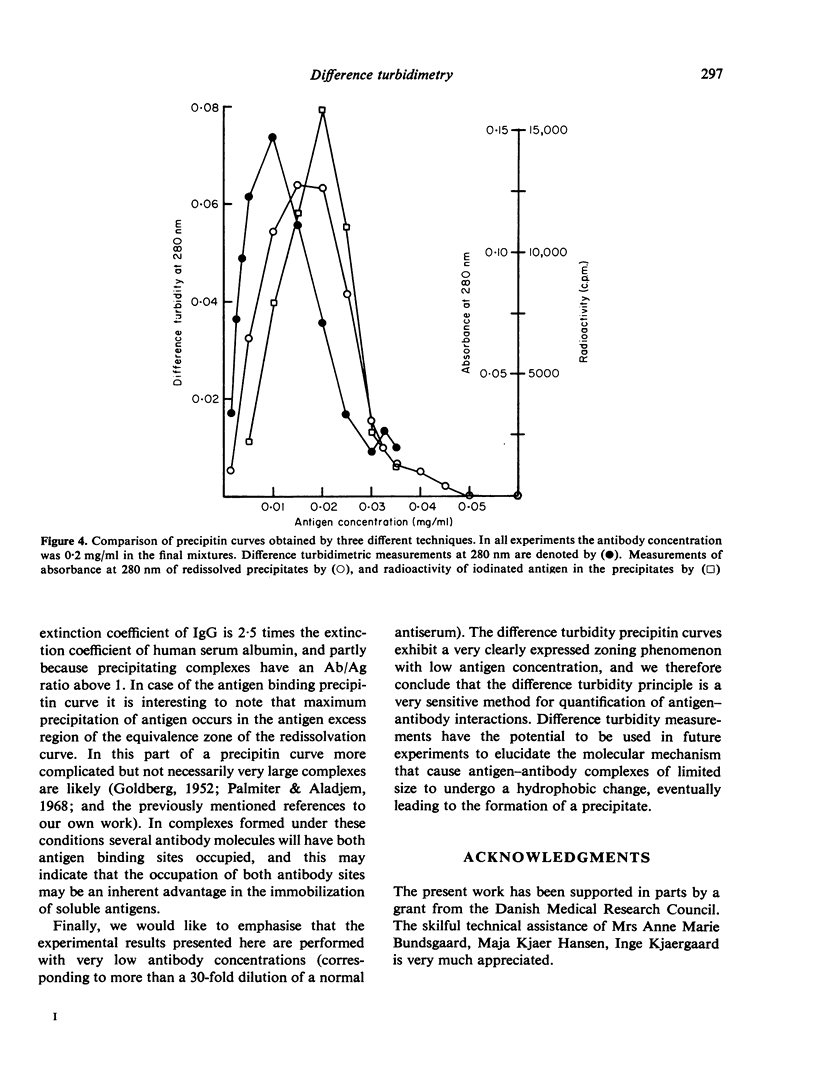
A new method for the measurement of precipitin and flocculation reactions between antibodies and antigens has been developed. The technique, called difference turbidimetry, involves the use of tandem cuvettes providing the opportunity of using separated and unmixed antigen and antibody as blank solutions for spectrophotometric readings in the ultraviolet wavelength range. By use of this technique genuine difference turbidity spectra have been recorded for the reaction between human serum albumin and rabbit-anti-human serum albumin IgG. It was found that difference turbidimetry at low wavelengths (e.g. 280 nm) allows the construction of precipitin curves with a very clearly expressed zoning phenomenon at a sensitivity which in terms of antigen and antibody concentrations is more than twice the sensitivity of conventional procedures. It is of special interest that the zone of equivalence differs when the same reaction between an antigen and its antibody is measured by difference turbidimetry, by absorbance of washed and redissolved precipitate, and by amount of precipitated antigen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A. A., Salahuddin A. Effects of pH, ionic strength and temperature on the ovalbuminanti-ovalbumin precipitin reaction. Immunology. 1973 Sep;25(3):377–383. [PMC free article] [PubMed] [Google Scholar]
- Davies G. E. The use of a sensitive micronephelometer in the estimation of antigens and precipitating antibodies. Immunology. 1971 May;20(5):779–787. [PMC free article] [PubMed] [Google Scholar]
- Hill R. J., Steensgaard J. Zonal centrifugation of soluble immune complexes. 2. Analysis of antigen-antibody mixtures at equilibrium over various parts of the precipitin curve. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(4):449–458. [PubMed] [Google Scholar]
- Jacobsen C. Covalent coupling of bilirubin to albumin. Int J Pept Protein Res. 1976;8(3):295–303. doi: 10.1111/j.1399-3011.1976.tb02507.x. [DOI] [PubMed] [Google Scholar]
- Palmiter M. T., Aladjem F. On the composition of insoluble antigen-antibody complexes. J Theor Biol. 1968 Jan;18(1):34–52. doi: 10.1016/0022-5193(68)90169-0. [DOI] [PubMed] [Google Scholar]
- Steensgaard J., Johansen H. K., Moller N. P. Computer simulation of immunochemical interactions. Immunology. 1975 Sep;29(3):571–579. [PMC free article] [PubMed] [Google Scholar]
- Steensgaard J., Liu B. M., Cline G. B., Møller N. P. The properties of immune complex-forming systems. A new theoretical approach. Immunology. 1977 Apr;32(4):445–456. [PMC free article] [PubMed] [Google Scholar]
- Vincent W. F., Harris E. W., Yaverbaum S. The estimation of precipitating antibody using a turbidimetric technique. Immunology. 1970 Feb;18(2):143–147. [PMC free article] [PubMed] [Google Scholar]


