Abstract
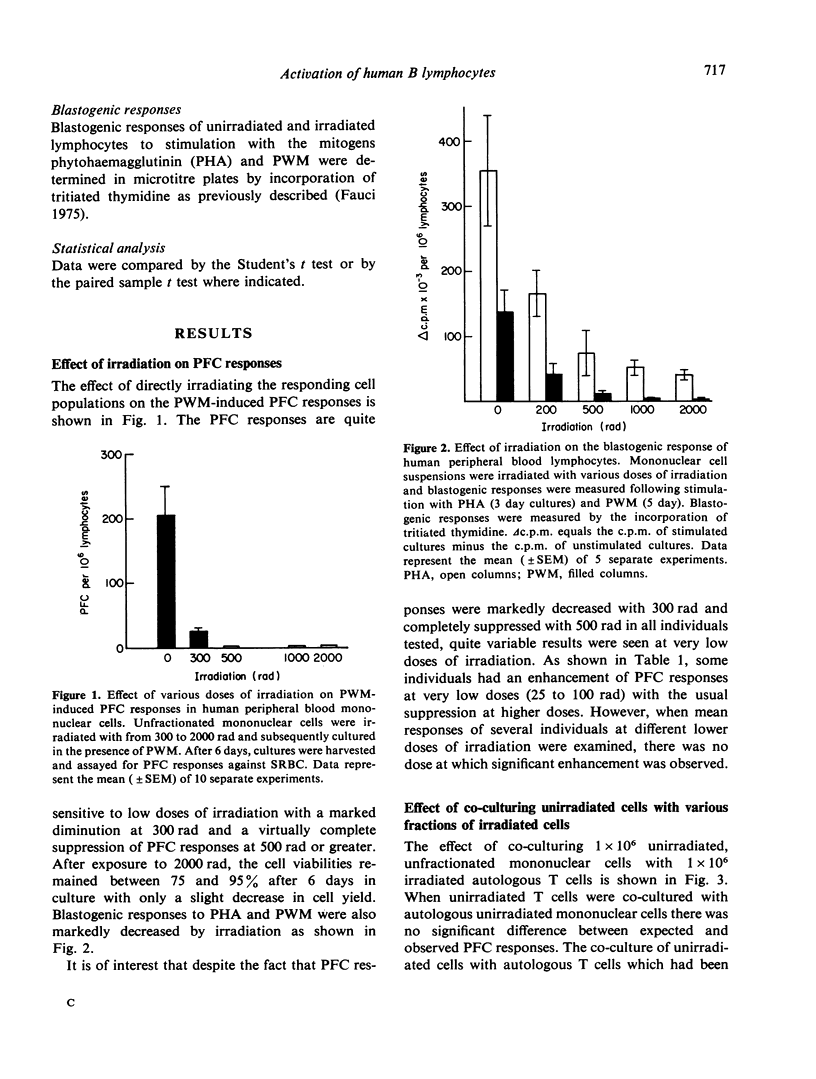
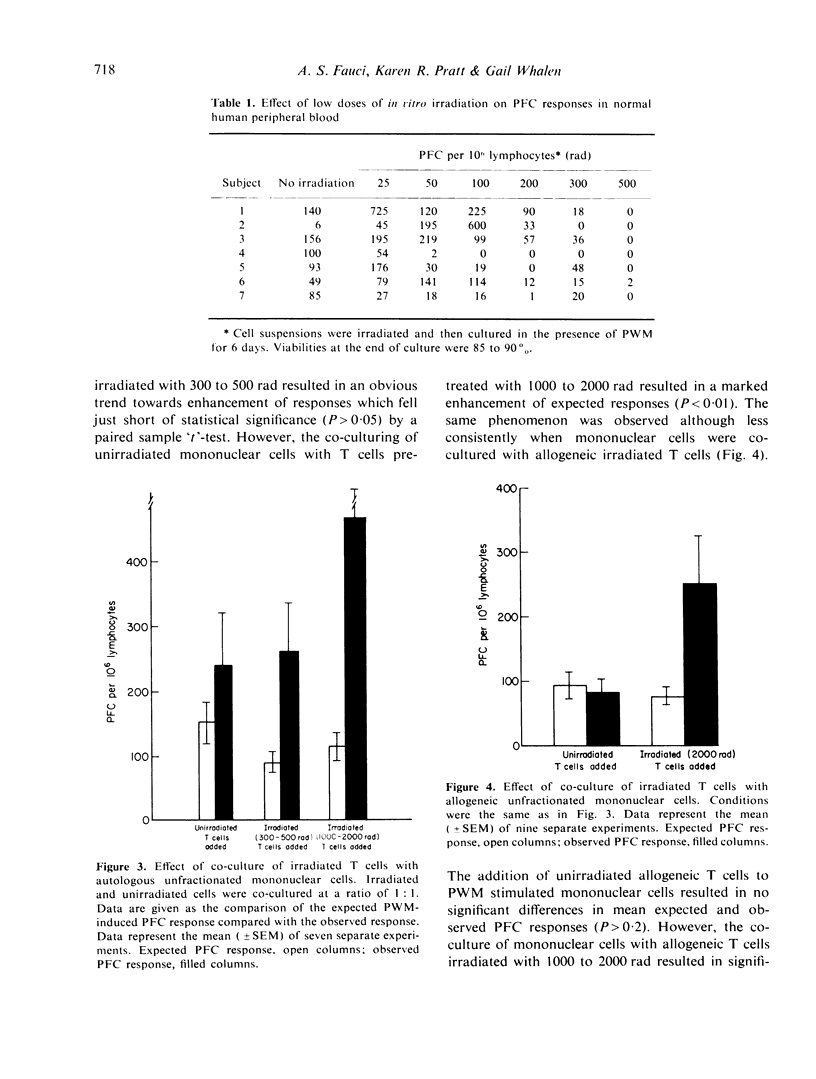
The differential effect of various doses of irradiation on subpopulations of human peripheral blood lymphoid cells involved in the pokeweed mitogen (PWM) induced PFC response against sheep red blood cells (SRBC) was studied. The plaque forming B cells were quite sensitive to low doses of irradiation with complete suppression of responses at 300 to 500 rad. On the contrary, helper T-cell function was resistant to 2000 rad. Co-culture of irradiated T cells with autologous or allogeneic B cells resulted in marked enhancement of PFC responses consistent with the suppression of naturally occurring suppressor cells with a resulting pure helper effect. Irradiated T-cell-depleted suspensions failed to produce this effect as did heat killed T cells, whereas mitomycin C treated T cells gave effects similar to irradiated T cells. These findings are consistent with a lack of requirement of cell division for a T-cell helper effect and a requirement of mitosis or another irradiation sensitive, mitomycin C sensitive process for a T-suppressor cell effect. These studies have potential relevance in the evaluation of subpopulations of human lymphoid cells involved in antibody production in normal individuals and in disease states.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Warner N. L. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Human bone marrow lymphocytes. I. Distribution of lymphocyte subpopulations in the bone marrow of normal individuals. J Clin Invest. 1975 Jul;56(1):98–110. doi: 10.1172/JCI108085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Activation of human B lymphocytes. I. Direct plaque-forming cell assay for the measurement of polyclonal activation and antigenic stimulation of human B lymphocytes. J Exp Med. 1976 Sep 1;144(3):674–684. doi: 10.1084/jem.144.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Polyclonal activation of bone-marrow-derived lymphocytes from human peripheral blood measured by a direct plaque-forming cell assay. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3676–3679. doi: 10.1073/pnas.73.10.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R., Whalen G. Activation of human B lymphocytes. II. Cellular interactions in the PFC response of human tonsillar and peripheral blood B lymphocytes to polyclonal activation by pokeweed mitogen. J Immunol. 1976 Dec;117(6):2100–2104. [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R., Whalen G. Activation of human B lymphocytes. IV. Regulatory effects of corticosteroids on the triggering signal in the plaque-forming cell response of human peripheral blood B lymphocytes to polyclonal activation. J Immunol. 1977 Aug;119(2):598–603. [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. III. Concanavalin A-induced generation of suppressor cells of the plaque-forming cell response of normal human B lymphocytes. J Immunol. 1977 Jun;118(6):2281–2287. [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Tse H. Y., Dutton R. W. Separation of helper and suppressor T lymphocytes. II. Ly phenotypes and lack of DNA synthesis requirement for the generation of concanavalin A helper and suppressor cells. J Exp Med. 1977 Sep 1;146(3):747–758. doi: 10.1084/jem.146.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Suppressor cells in the regulation of the immune response. Prog Clin Immunol. 1977;3:155–199. [PubMed] [Google Scholar]



