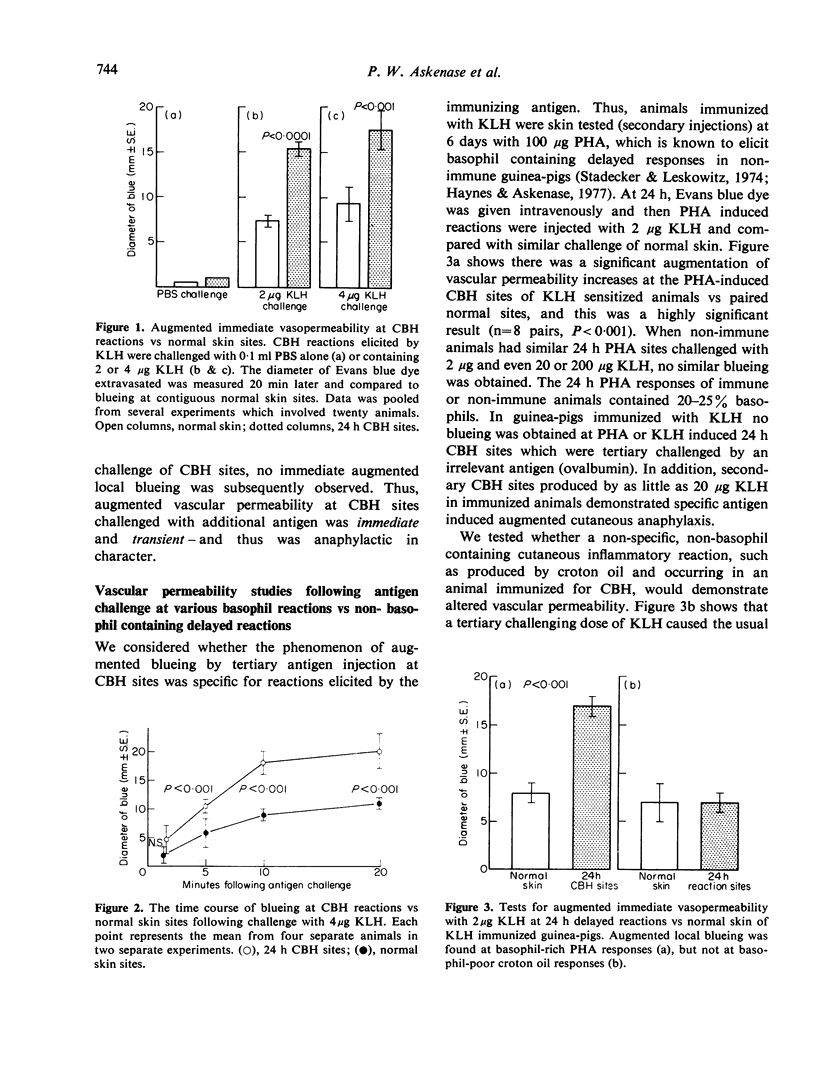
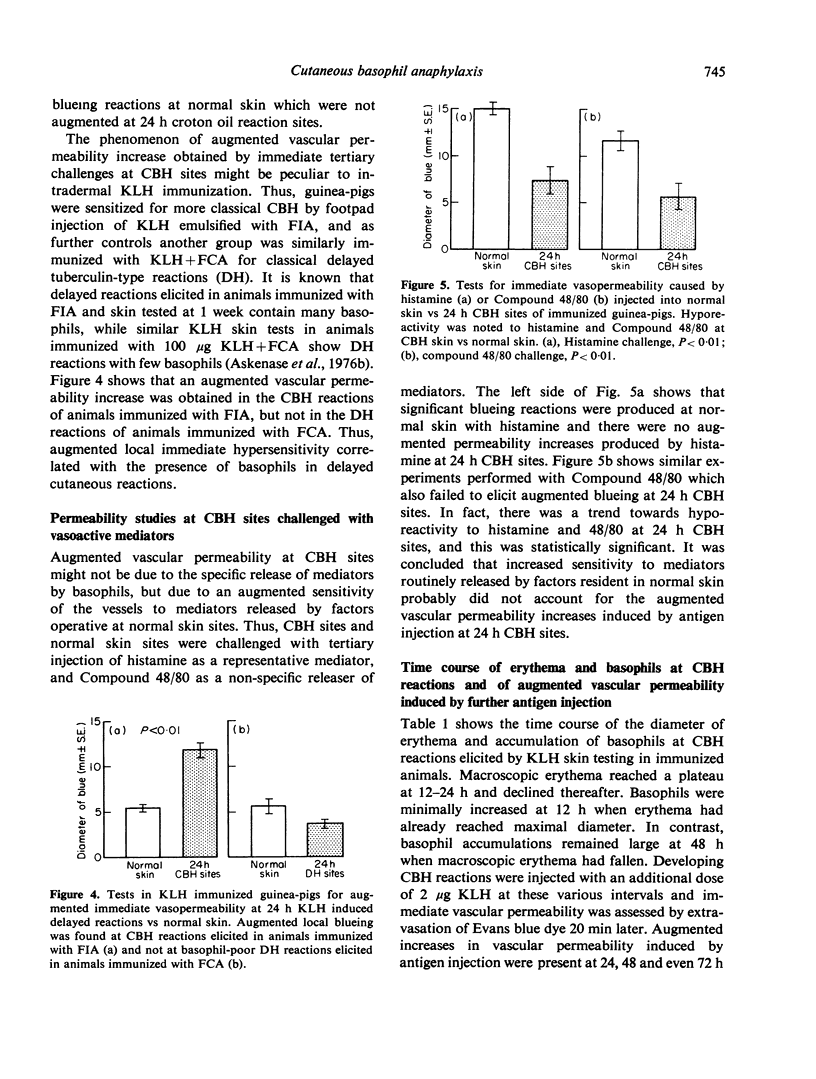
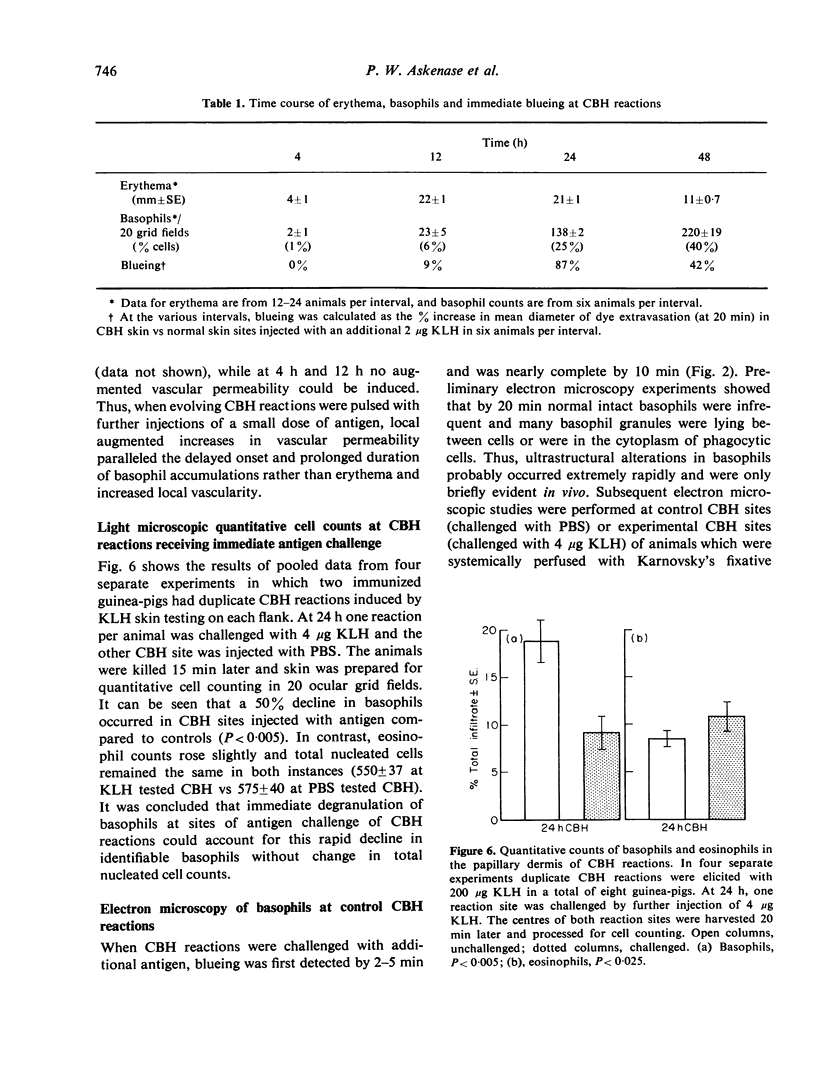
Abstract
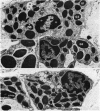
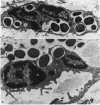
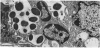
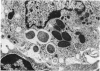
Many delayed-type reactions contain large infiltrates of basophils whose function is unknown. We have studied these cutaneous basophil hypersensitivity (CBH) reactions in guinea-pigs to ascertain whether basophils that are recruited to delayed reaction sites could be triggered for immediate reactivity. We compared 24 h CBH reactions with nearby skin for immediate hypersensitivity by challenging each site with small amounts of antigen. CBH sites had augmented immediate increases in vascular permeability detected by extravasation of Evan's blue dye. The ability to elicit this augmented anaphylactic phenomenon correlated with the local presence of basophils, and light microscopy at CBH reactions 15 min after antigen challenge showed a 50% decline in basophil counts. Electron microscopy showed that progressive anaphylactic-type degranulation of local basophils occurred within minutes following reintroduction of antigen. There was fusion of vacuoles containing granules, exocytosis of granules, and dissolution of granules, without ultrastructural disruption of cellular integrity. These results establish that basophils in CBH reactions can be triggered with soluble antigen to undergo anaphylactic degranulation, with the immediate release of vasoactive mediators. We have termed this phenomenon 'cutaneous basophil anaphylaxis'. Thus, one function of basophils at sites of delayed hypersensitivity may be to provide the potential for augmented, local, immediate anaphylactic reactivity.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenase P. W. Cutaneous basophil hypersensitivity uncovered in the cell transfer of classical tuberculin hypersensitivity. J Immunol. 1976 Sep;117(3):741–747. [PubMed] [Google Scholar]
- Askenase P. W. Role of basophils, mast cells, and vasoamines in hypersensitivity reactions with a delayed time course. Prog Allergy. 1977;23:199–320. [PubMed] [Google Scholar]
- Colvin R. B., Dvorak H. F. Role of the clotting system in cell-mediated hypersensitivity. II. Kinetics of fibrinogen/fibrin accumulation and vascular permeability changes in tuberculin and cutaneous basophil hypersensitivity reactions. J Immunol. 1975 Jan;114(1 Pt 2):377–387. [PubMed] [Google Scholar]
- Dvorak A. M., Dvorak H. F., Karnovsky M. J. Uptake of horseradish peroxidase by guinea pig basophilic leukocytes. Lab Invest. 1972 Jan;26(1):27–39. [PubMed] [Google Scholar]
- Dvorak A. M., Mihm M. C., Jr, Dvorak H. F. Degranulation of basophilic leukocytes in allergic contact dermatitis reactions in man. J Immunol. 1976 Mar;116(3):687–695. [PubMed] [Google Scholar]
- Dvorak H. F., Colvin R. B., Churchill W. H. Specificity of basophils and lymphocytes in cutaneous basophil hypersensitivity. J Immunol. 1975 Jan;114(1 Pt 2):507–511. [PubMed] [Google Scholar]
- Dvorak H. F., Dvorak A. M., Churchill W. H. Immunologic rejection of diethylnitrosamine-induced hepatomas in strain 2 guinea pigs: participation of basophilic leukocytes and macrophage aggregates. J Exp Med. 1973 Mar 1;137(3):751–775. doi: 10.1084/jem.137.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Dvorak A. M., Simpson B. A., Richerson H. B., Leskowitz S., Karnovsky M. J. Cutaneous basophil hypersensitivity. II. A light and electron microscopic description. J Exp Med. 1970 Sep 1;132(3):558–582. doi: 10.1084/jem.132.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Mihm M. C., Jr, Dvorak A. M. Morphology of delayed-type hypersensitivity reactions in man. J Invest Dermatol. 1976 Sep;67(3):391–401. doi: 10.1111/1523-1747.ep12514713. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Simpson B. A., Bast R. C., Jr, Leskowitz S. Cutaneous basophil hypersensitivity. 3. Participation of the basophil in hypersensitivity to antigen-antibody complexes, delayed hypersensitivity and contact allergy. Passive transfer. J Immunol. 1971 Jul;107(1):138–148. [PubMed] [Google Scholar]
- Evans R., Pence H., Kaplan H., Rocklin R. E. The effect of immunotherapy on humoral and cellular responses in ragweed hayfever. J Clin Invest. 1976 May;57(5):1378–1385. doi: 10.1172/JCI108406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Askenase P. W., Gershon M. D. Requirement for vasoactive amines for production of delayed-type hypersensitvity skin reactions. J Exp Med. 1975 Sep 1;142(3):732–747. doi: 10.1084/jem.142.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie R., Levy D. A., Weiss L. The antigen-induced degranulation of basophil leukocytes from atopic subjects studied by electron microscopy. Lab Invest. 1977 Feb;36(2):173–182. [PubMed] [Google Scholar]
- Hastie R. The antigen-induced degranulation of basophil leucocytes from atopic subjects, studied by phase-contrast microscopy. Clin Exp Immunol. 1971 Jan;8(1):45–61. [PMC free article] [PubMed] [Google Scholar]
- Haynes J. D., Askenase P. W. Cuta neous basophil responses in neonatal guinea pigs: active immunization, hapten specific transfer with small amounts of serum, and preferential elicitation with phytohemagglutinin skin testing. J Immunol. 1977 Mar;118(3):1063–1069. [PubMed] [Google Scholar]
- Huxtable C. R., Rothwell T. L. Studies of the responses of basophil and eosinophil leucocytes and mast cells to the nematode Trichostrongylus colubriformis. Ultrastructural changes in basophils and eosinophils at the site of infection. Aust J Exp Biol Med Sci. 1975 Dec;53(6):437–445. doi: 10.1038/icb.1975.49. [DOI] [PubMed] [Google Scholar]
- Norman P. S., Lichtenstein L. M. Capacity of purified antigens and whole pollen extracts to release histamine from leukocytes of hay fever patients. J Allergy Clin Immunol. 1973 Aug;52(2):94–104. doi: 10.1016/0091-6749(73)90081-x. [DOI] [PubMed] [Google Scholar]
- Orange R. P. Immunopharmacological aspects of bronchial asthma. Clin Allergy. 1973 Dec;3 (Suppl):521–537. doi: 10.1111/j.1365-2222.1973.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Askenase P. W., Gershon R. K. The effect of locally injected vasoactive amines on the elicitation of delayed-type hypersensitivity. J Immunol. 1977 Jan;118(1):159–165. [PubMed] [Google Scholar]
- Soter N. A., Austen K. F. The diversity of mast cell-derived mediators: implications for acute, subacute, and chronic cutaneous inflammatory disorders. J Invest Dermatol. 1976 Sep;67(3):313–319. doi: 10.1111/1523-1747.ep12514349. [DOI] [PubMed] [Google Scholar]
- Stadecker M. J., Leskowitz S. The cutaneous basophil response to mitogens. J Immunol. 1974 Aug;113(2):496–500. [PubMed] [Google Scholar]
- Terry R. W., Bainton D. F., Farquhar M. G. Formation and structure of specific granules in basophilic leukocytes of the guinea pig. Lab Invest. 1969 Jul;21(1):65–76. [PubMed] [Google Scholar]
- Williams T. J., Morley J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature. 1973 Nov 23;246(5430):215–217. doi: 10.1038/246215a0. [DOI] [PubMed] [Google Scholar]