Abstract
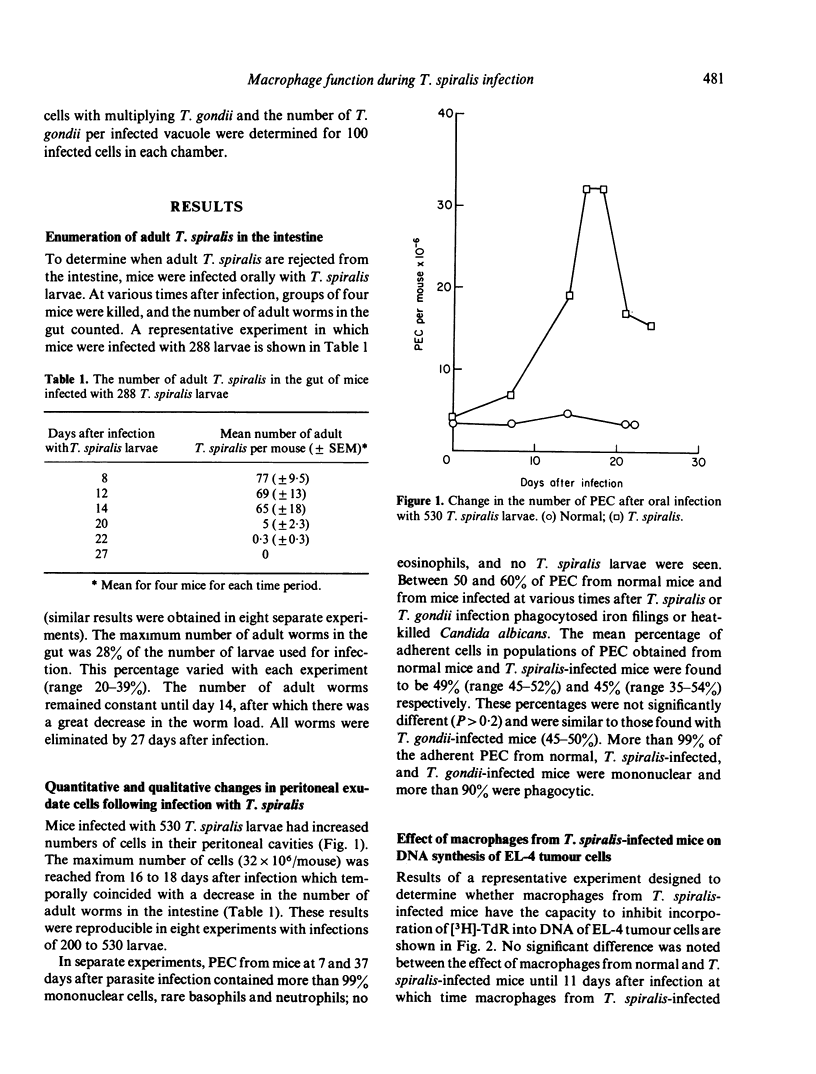
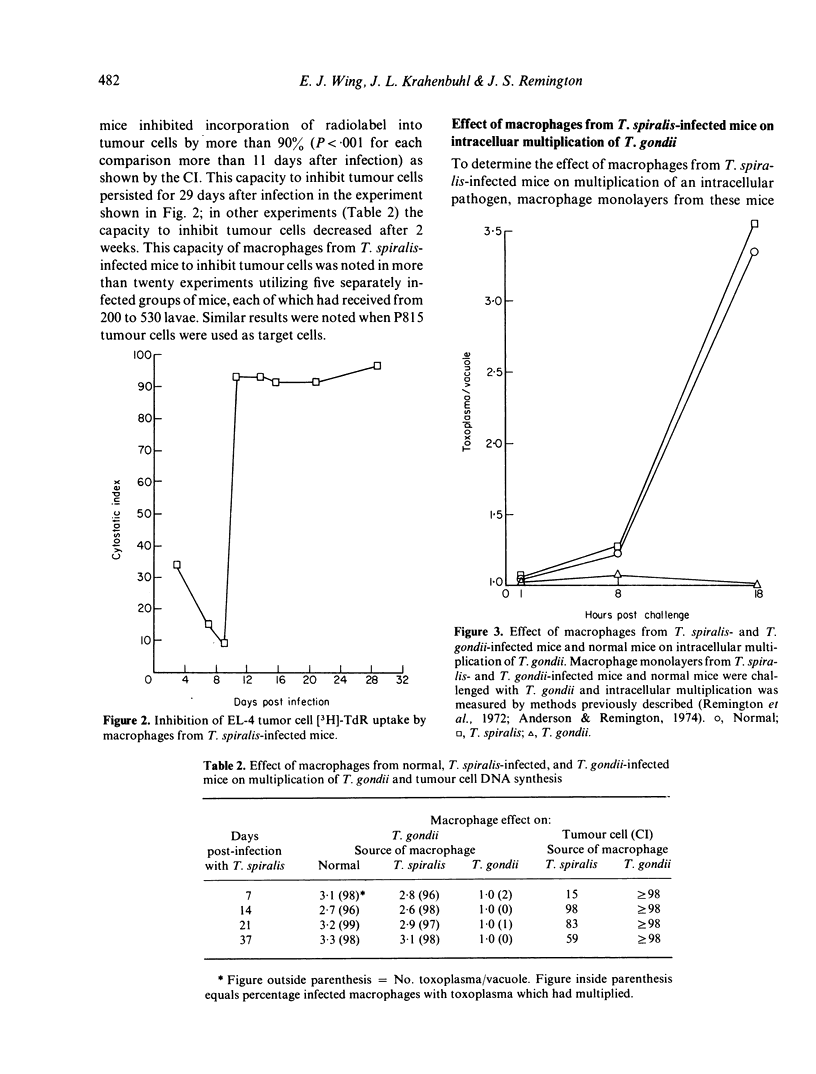
Studies were made to investigate the quantitative and functional changes which occur in peritoneal macrophage populations obtained from mice infected orally with Trichinella spiralis larvae. C57BL/6 mice infected with T. spiralis larvae became parasitized with adult worms which were rejected from the intestine from 14 to 20 days after infection. Infected mice developed a striking increase in peritoneal exudate cells, composed largely of macrophages, which was maximal at from 16 to 18 days after infection. T. spiralis larvae and eosinophils were not seen in the peritoneal exudates. Macrophages from mice infected more than 11 days earlier inhibited DNA synthesis of syngeneic and allogeneic tumour cells, a property atributed to activated macrophages. In addition, macrophages from T. spiralis-infected mice had the functional ability to kill EL-4 tumour cells as measured by 51Cr release. Unlike activated macrophages, however, macrophages from infected mice did not develop the ability to inhibit multiplication of the intracellular pathogen Toxoplasma gondii. These studies demonstrate that T. spiralis infection in mice induces changes in macrophage function that differ from changes associated with infections by intracellular pathogens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. E., Jr, Remington J. S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974 May 1;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Gardner I. D., Remington J. S. Age-related decline in the resistance of mice to infection with intracellular pathogens. Infect Immun. 1977 May;16(2):593–598. doi: 10.1128/iai.16.2.593-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffar A., Cullen R. T., Dunbar N., Woodruff M. F. Anti-tumour effect in vitro of lymphocytes and macrophages from mice treated with Corynebacterium parvum. Br J Cancer. 1974 Mar;29(3):199–205. doi: 10.1038/bjc.1974.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Control of carcinogenesis: a possible role for the activated macrophage. Science. 1972 Sep 15;177(4053):998–1000. doi: 10.1126/science.177.4053.998. [DOI] [PubMed] [Google Scholar]
- Kaplan A. M., Morahan P. S., Regelson W. Induction of macrophage-mediated tumor-cell cytotoxicity by pyran copolymer. J Natl Cancer Inst. 1974 Jun;52(6):1919–1923. doi: 10.1093/jnci/52.6.1919. [DOI] [PubMed] [Google Scholar]
- Keller R. Cytostatic elimination of syngeneic rat tumor cells in vitro by nonspecifically activated macrophages. J Exp Med. 1973 Sep 1;138(3):625–644. doi: 10.1084/jem.138.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Jones V. E. Role of activated macrophages and antibody in inhibition and enhancement of tumour growth in rats. Lancet. 1971 Oct 16;2(7729):847–849. doi: 10.1016/s0140-6736(71)90222-4. [DOI] [PubMed] [Google Scholar]
- Larsh J. E., Jr Allergic inflammation as a hypothesis for the expulsion of worms from tissues: a review. Exp Parasitol. 1975 Apr;37(2):251–266. doi: 10.1016/0014-4894(75)90077-6. [DOI] [PubMed] [Google Scholar]
- Larsh J. E., Jr, Race G. J., Goulson H. T., Weatherly N. F. Studies on delayed (cellular) hypersensitivity in mice infected with Trichinella spiralis. 3. Serologic and histopathologic findings in recipients given peritoneal exudate cells. J Parasitol. 1966 Feb;52(1):146–156. [PubMed] [Google Scholar]
- Lee K. C., Berry D. Functional heterogeneity in macrophages activated by Corynebacterium parvum: characterization of subpopulations with different activities in promoting immune responses and suppressing tumor cell growth. J Immunol. 1977 May;118(5):1530–1540. [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch E., Bomford R. Macrophage potentiation by Trichinella spiralis. Ann Trop Med Parasitol. 1977 Jun;71(2):245–248. doi: 10.1080/00034983.1977.11687187. [DOI] [PubMed] [Google Scholar]
- Olivotto M., Bomford R. In vitro inhibition of tumour cell growth and DNA synthesis by peritoneal and lung macrophages from mice injected with Corynebacterium parvum. Int J Cancer. 1974 Apr 15;13(4):478–488. doi: 10.1002/ijc.2910130406. [DOI] [PubMed] [Google Scholar]
- Pelley R. P., Karp R., Mahmoud A. A., Warren K. S. Antigen dose response and specificity of production of the lymphokine eosinophil stimulation promoter. J Infect Dis. 1976 Sep;134(3):230–237. doi: 10.1093/infdis/134.3.230. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Krahenbuhl J. L., Mendenhall J. W. A role for activated macrophages in resistance to infection with Toxoplasma. Infect Immun. 1972 Nov;6(5):829–834. doi: 10.1128/iai.6.5.829-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. G., Fishman M. Functional and morphological heterogeneity among rabbit peritoneal macrophages. Cell Immunol. 1974 Mar 30;11(1-3):130–145. doi: 10.1016/0008-8749(74)90014-8. [DOI] [PubMed] [Google Scholar]
- Ruskin J., McIntosh J., Remington J. S. Studies on the mechanisms of resistance to phylogenetically diverse intracellular organisms. J Immunol. 1969 Aug;103(2):252–259. [PubMed] [Google Scholar]
- Stadecker M. J., Calderon J., Karnovsky M. L., Unanue E. R. Synthesis and release of thymidine by macrophages. J Immunol. 1977 Nov;119(5):1738–1743. [PubMed] [Google Scholar]
- Swartzberg J. E., Krahenbuhl J. L., Remington J. S. Dichotomy between macrophage activation and degree of protection against Listeria monocytogenes and Toxoplasma gondii in mice stimulated with Corynebacterium parvum. Infect Immun. 1975 Nov;12(5):1037–1043. doi: 10.1128/iai.12.5.1037-1043.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. S. Functional heterogeneity of macrophages in the induction and expression of acquired immunity. J Reticuloendothel Soc. 1976 Jul;20(1):57–65. [PubMed] [Google Scholar]
- Weatherly N. F. Increased survival of Swiss mice given sublethal infections of Trichinella spiralis. J Parasitol. 1970 Aug;56(4):748–752. [PubMed] [Google Scholar]
- Wing E. J., Gardner I. D., Ryning F. W., Remington J. S. Dissociation of effector functions in populations of activated macrophages. Nature. 1977 Aug 18;268(5621):642–644. doi: 10.1038/268642a0. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Remington J. S. Studies on the regulation of lymphocyte reactivity by normal and activated macrophages. Cell Immunol. 1977 Apr;30(1):108–121. doi: 10.1016/0008-8749(77)90052-1. [DOI] [PubMed] [Google Scholar]



