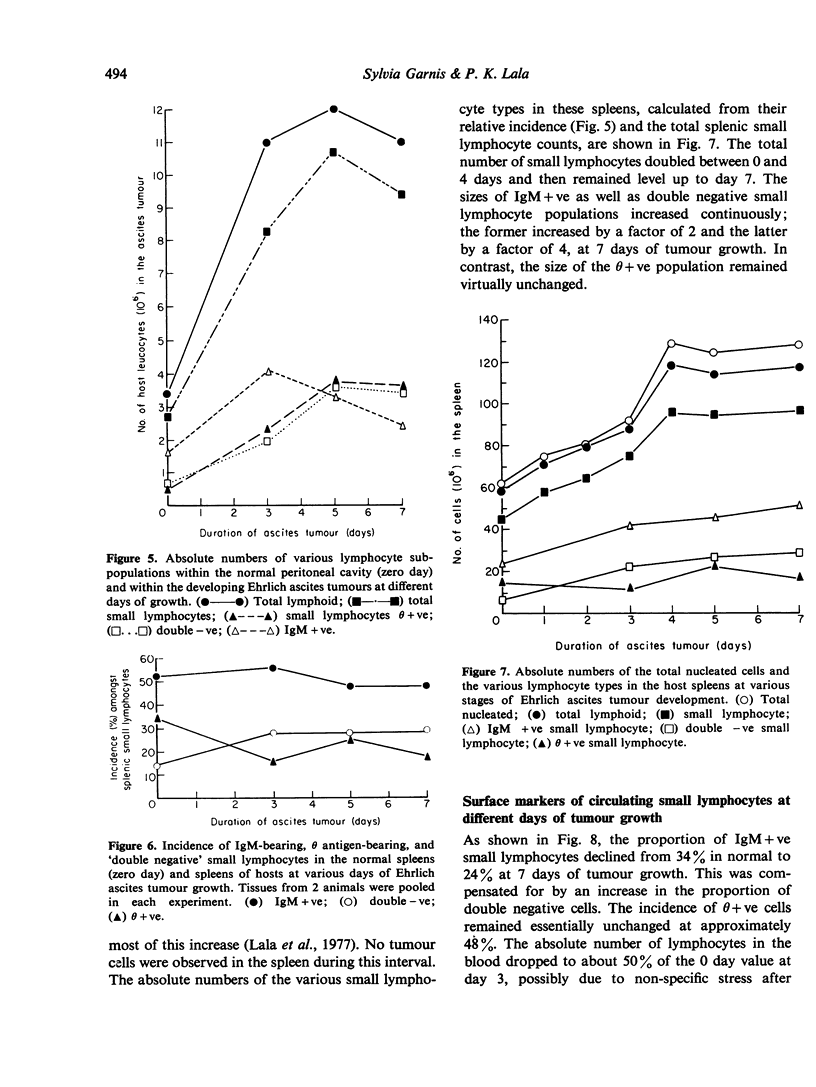
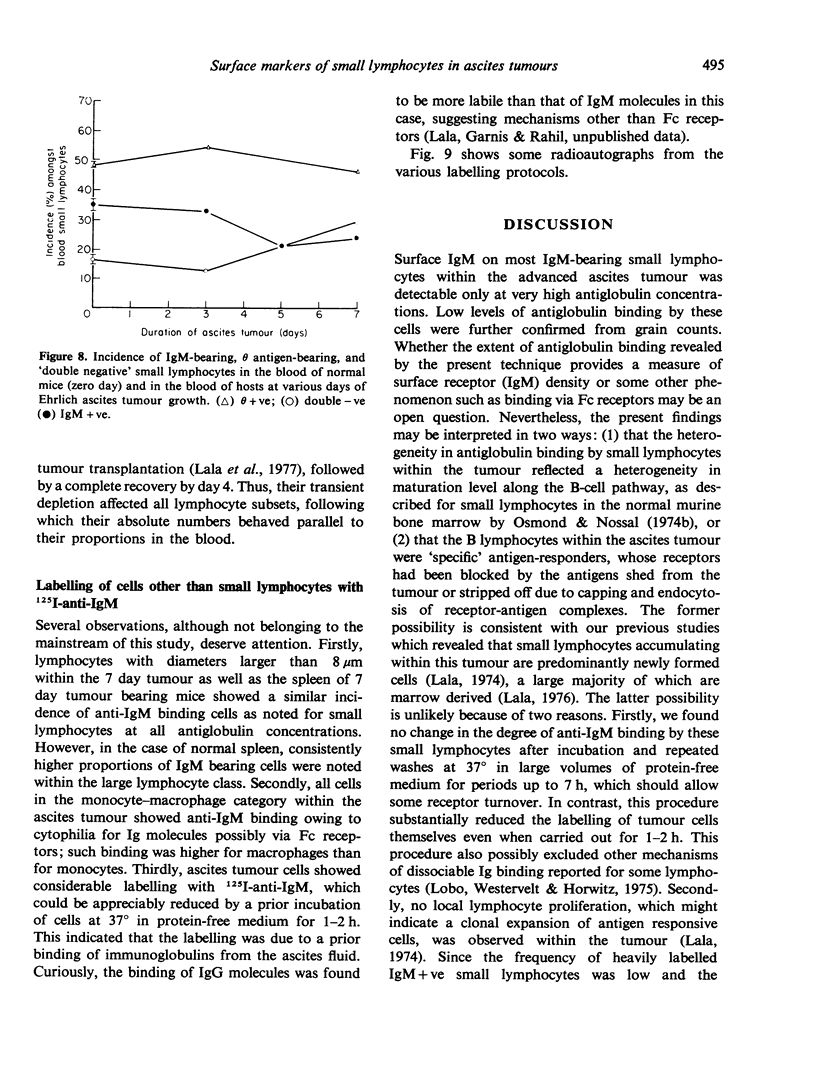
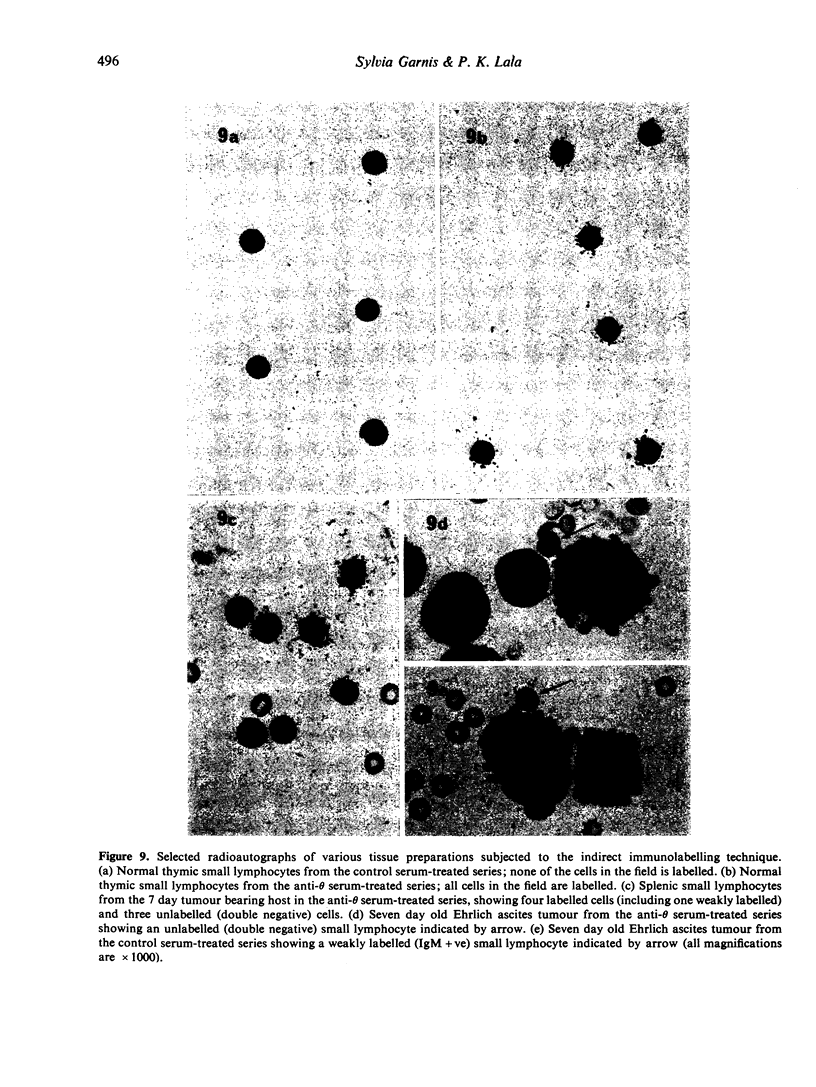
Abstract
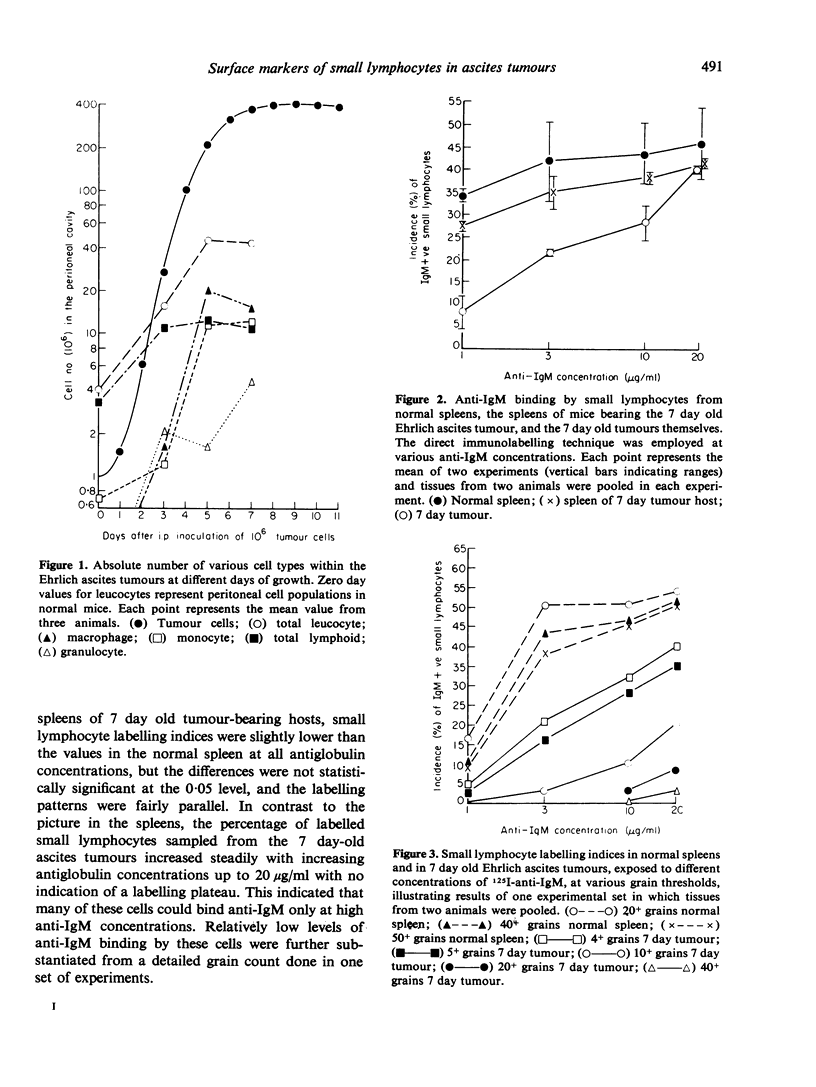
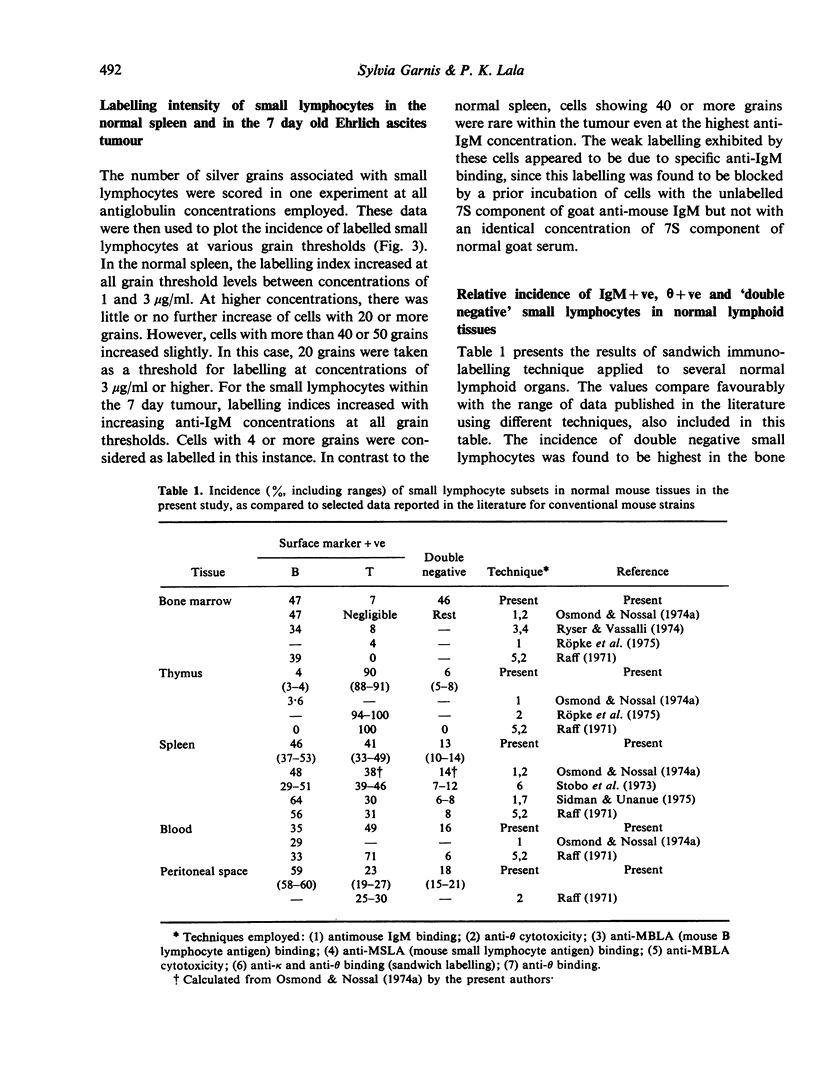
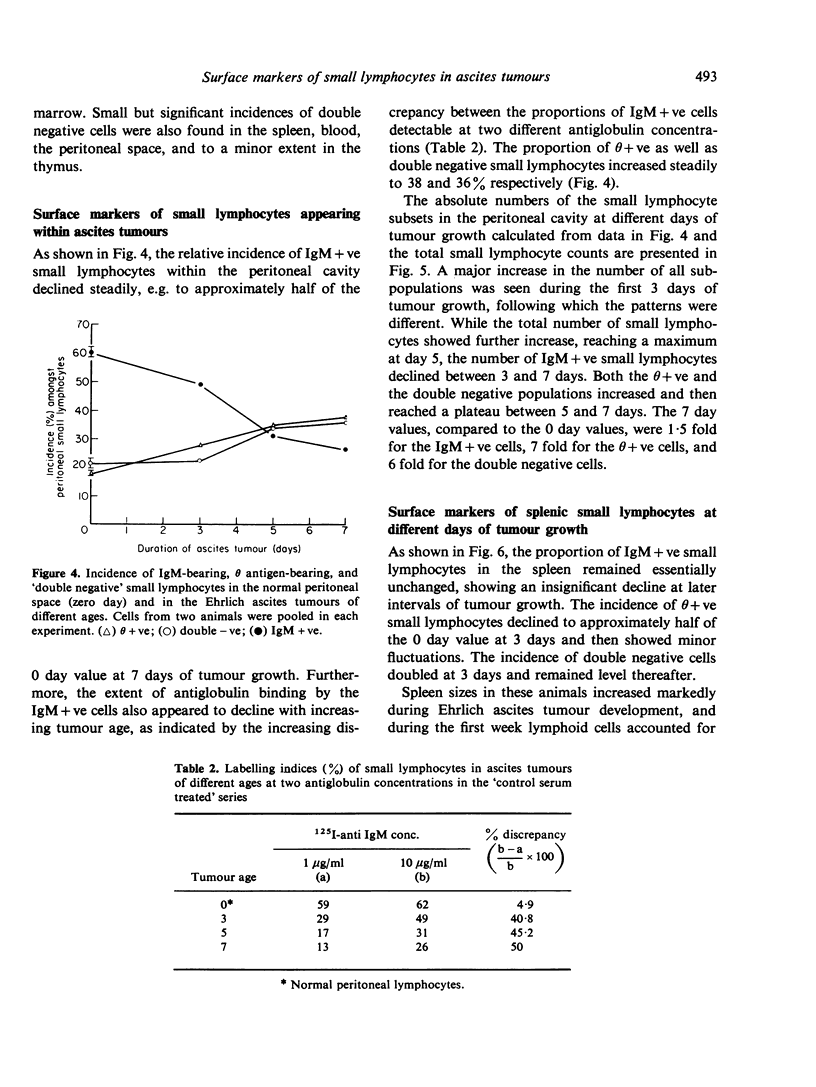
Small lymphocytes sampled from intraperitoneally growing Ehrlich ascites tumour in CBA/H-T6 mice as well as host spleen and blood at different days of tumour development were characterized radioautographically on the basis of two surface markers, IgM for B cells and θ antigen for T cells. A direct binding of 125I-labelled anti-IgM detected natural surface IgM, while an indirect binding following a prior exposure to anti-θ antibody detected θ antigen. Cells remaining unlabelled with the latter procedure were considered to lack both markers (double negative). While the incidence of IgM+ve small lymphocytes within the tumour declined, their absolute numbers increased with tumour growth. Low levels of antiglobulin binding shown by these cells were considered to reflect low levels of maturation, because (1) our previous studies indicated that they were newly formed, and (2) the extent of antiglobulin binding by B lymphocytes in the marrow is known to increase with increasing post-mitotic age. The proportions and the absolute numbers of θ+ve as well as the double negative small lymphocytes increased within the growing tumours. Within the host spleen, the incidence of IgM+ve small lymphocytes remained unchanged but their absolute numbers increased because of splenomegaly. The degree of antiglobulin binding by these cells was comparable to that of the normal splenic population. The incidence of θ+ve cells dropped but their absolute numbers remained unchanged in the spleen during tumour growth. In contrast, the incidence as well as the absolute numbers of double negative cells increased markedly. This cell category increased also in the blood, possibly in transit to the tumour site and other lymphoid organs from the bone marrow, where they were most prevalent. Their bone marrow origin was further suggested by a preponderance of marrow derived small lymphocytes at the tumour site as well as in the host spleens found in our earlier studies. Double negative population in the spleen showed a paucity of C′3 and Fc receptors on the cell surface and included cells capable of producing B lymphoid colonies in vitro.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Hämmerling U., De Harven E., Boyse E. A., Old L. J. Antigenic structure of cell surfaces. An immunoferritin study of the occurrence and topography of H-2' theta, and TL alloantigens on mouse cells. J Exp Med. 1969 Nov 1;130(5):979–1001. doi: 10.1084/jem.130.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaakov M., Haran-Ghera N. T & B lymphocytes in thymus of SJL/J mice. Nature. 1975 May 1;255(5503):64–66. doi: 10.1038/255064a0. [DOI] [PubMed] [Google Scholar]
- Bernard J., Jeannesson P., Zagury D., Fridman W. H., Ternynck T., Avrameas S. Biology of isolated immunocytes. II. Simultaneous detection of cell surface Ig and theta-antigen by immunoperoxidase staining at the ultrastructural level. Ann Immunol (Paris) 1975 Oct-Dec;126(5-6):565–580. [PubMed] [Google Scholar]
- Blomgren H., Glas U., Franzén S., Granberg P. O. Lymphoid cells in carcinoma of the breast. Failure of response to phytohaemagglutinin in vitro. Acta Radiol Ther Phys Biol. 1973 Oct;12(5):434–442. doi: 10.3109/02841867309130410. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., McCoy J. L., Cannon G. B., Reeves W. J., West W. H., Hergerman R. B. Lymphocytes forming rosettes with sheep erythrocytes in metastatic pleural effusions. J Natl Cancer Inst. 1976 May;56(5):1051–1052. doi: 10.1093/jnci/56.5.1051. [DOI] [PubMed] [Google Scholar]
- Dorizzi M., Ortiz-Muniz G., Lopez D. M., Sigel M. M., Epstein R. S. Increase in the proportion of cells with the C'3 receptor in BALB/c mice bearing mammary tumors. Int J Cancer. 1975 Dec 15;16(6):1015–1021. doi: 10.1002/ijc.2910160615. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershwin M. E., Chused T. M., Steinberg A. D. Cytotoxic activity of anti-theta antibody found in the gamma m fraction. Transplantation. 1974 Oct;18(4):377–380. doi: 10.1097/00007890-197410000-00015. [DOI] [PubMed] [Google Scholar]
- Greenberg A. H., Hudson L., Shen L., Roitt I. M. Antibody-dependent cell-mediated cytotoxicity due to a "null" lymphoid cell. Nat New Biol. 1973 Mar 28;242(117):111–113. doi: 10.1038/newbio242111a0. [DOI] [PubMed] [Google Scholar]
- Haskill J. S., Proctor J. W., Yamamura Y. Host responses with solid tumors. I. Monocytic effector cells within rat sarcomas. J Natl Cancer Inst. 1975 Feb;54(2):387–393. [PubMed] [Google Scholar]
- Haskill J. S., Yamamura Y., Radov L. Host responses within solid tumors: non-thymus-derived specific cytotoxic cells within a murine mammary adenocarcinoma. Int J Cancer. 1975 Nov 15;16(5):798–809. doi: 10.1002/ijc.2910160512. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Kaizer L., Lala P. K. Post-mitotic age of monocuclear cells migrating into TA-3(St) solid tumors. Cell Tissue Kinet. 1977 May;10(3):279–288. doi: 10.1111/j.1365-2184.1977.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Pross H. F., Elliott E. V. Origin and partial characterization of Fc receptor-bearing cells found within experimental carcinomas and sarcomas. Int J Cancer. 1975 Jun 15;15(6):918–932. doi: 10.1002/ijc.2910150607. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Chused T. M., Herberman R. B., Holden H. T., Lavrin D. H. Evidence of suppressor cell activity in spleens of mice bearing primary tumors induced by Moloney sarcoma virus. J Exp Med. 1974 Jun 1;139(6):1473–1487. doi: 10.1084/jem.139.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Muchmore A. V., Chused T. M., Holden H. T., Herberman R. B. Inhibition of proliferation of lymphoma cells and T lymphocytes by suppressor cells from spleens of tumor-bearing mice. J Immunol. 1975 Jan;114(1 Pt 1):206–210. [PubMed] [Google Scholar]
- Konda S., Nakao Y., Smith R. T. The stimulatory effect of tumor bearing upon the T- and B-cell subpopulations of the mouse spleen. Cancer Res. 1973 Oct;33(10):2247–2256. [PubMed] [Google Scholar]
- Lala P. K. Dynamics of leukocyte migration into the mouse ascites tumor. Cell Tissue Kinet. 1974 May;7(3):293–304. doi: 10.1111/j.1365-2184.1974.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Lala P. K., Kaizer L. Surface markers of small lymphocytes appearing in murine TA-3(St) solid tumors, host spleen, and blood. J Natl Cancer Inst. 1977 Jul;59(1):237–244. doi: 10.1093/jnci/59.1.237. [DOI] [PubMed] [Google Scholar]
- Lala P. K., Patt H. M. Cytokinetic analysis of tumor growth. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1735–1742. doi: 10.1073/pnas.56.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo P. I., Westervelt F. B., Horwitz D. A. Identification of two populations of immunoglobulin-bearing lymphocytes in man. J Immunol. 1975 Jan;114(1 Pt 1):116–119. [PubMed] [Google Scholar]
- Lopes Cardozo E., Harting M. C. On the function of lymphocytes in malignant effusions. Acta Cytol. 1972 Jul-Aug;16(4):307–313. [PubMed] [Google Scholar]
- Loring M., Schlesinger M. The theta antigenicity of lymphoid organs of mice bearing the Ehrlich ascites tumor. Cancer Res. 1970 Aug;30(8):2204–2207. [PubMed] [Google Scholar]
- Malka S., Oon C. J., Hobbs J. R. Excess IgD bearing lymphocytes in patients with malignant melanoma. Br J Cancer. 1974 Nov;30(5):379–381. doi: 10.1038/bjc.1974.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Nossal G. J., Warner N. L., Miller J. F., Mandel T. E., Layton J. E., Gutman G. A. Growth of B-lymphocyte colonies in vitro. J Exp Med. 1975 Dec 1;142(6):1534–1549. doi: 10.1084/jem.142.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nind A. P., Nairn R. C., Rolland J. M., Guli E. P., Hughes E. S. Lymphocyte anergy in patients with carcinoma. Br J Cancer. 1973 Aug;28(2):108–117. doi: 10.1038/bjc.1973.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H., Sprent J. Quantitative features of a sandwich radioimmunolabeling technique for lymphocyte surface receptors. J Exp Med. 1972 Feb 1;135(2):405–428. doi: 10.1084/jem.135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond D. G., Nossal G. J. Differentiation of lymphocytes in mouse bone marrow. I. Quantitative radioautographic studies of antiglobulin binding by lymphocytes in bone marrow and lymphoid tissues. Cell Immunol. 1974 Jul;13(1):117–131. doi: 10.1016/0008-8749(74)90232-9. [DOI] [PubMed] [Google Scholar]
- Osmond D. G., Nossal G. J. Differentiation of lymphocytes in mouse bone marrow. II. Kinetics of maturation and renewal of antiglobulin-binding cells studied by double labeling. Cell Immunol. 1974 Jul;13(1):132–145. doi: 10.1016/0008-8749(74)90233-0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., Parish C. R. Surface properties of cells involved in antibody-dependent cytotoxicity. Cell Immunol. 1976 Feb;21(2):226–235. doi: 10.1016/0008-8749(76)90051-4. [DOI] [PubMed] [Google Scholar]
- Russell S. W., Gillespie G. Y., Hansen C. B., Cochrane C. G. Inflammatory cells in solid murine neoplasms. II. Cell types found throughout the course of Moloney sarcoma regression or progression. Int J Cancer. 1976 Sep 15;18(3):331–338. doi: 10.1002/ijc.2910180310. [DOI] [PubMed] [Google Scholar]
- Ryser J. E., Vassalli P. Mouse bone marrow lymphocytes and their differentiation. J Immunol. 1974 Sep;113(3):719–728. [PubMed] [Google Scholar]
- Röpke C., Hougen H. P., Everett N. B. Long-lived T and B lymphocytes in the bone marrow and thoracic duct lymph of the mouse. Cell Immunol. 1975 Jan;15(1):82–93. doi: 10.1016/0008-8749(75)90166-5. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Unanue E. R. Development of B lymphocytes. I. Cell populations and a critical event during ontogeny. J Immunol. 1975 Jun;114(6):1730–1735. [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. V. Lymphocytes lacking detectable surface theta or immunoglobulin determinants. J Exp Med. 1973 Jul 1;138(1):71–88. doi: 10.1084/jem.138.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood J. C. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer. 1974 Dec;30(6):538–548. doi: 10.1038/bjc.1974.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loveren H., Den Otter W. Macrophages in solid tumors. I. Immunologically specific effector cells. J Natl Cancer Inst. 1974 Oct;53(4):1057–1060. doi: 10.1093/jnci/53.4.1057. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- van Furth R., Hirsch J. G., Fedorko M. E. Morphology and peroxidase cytochemistry of mouse promonocytes, monocytes, and macrophages. J Exp Med. 1970 Oct 1;132(4):794–812. doi: 10.1084/jem.132.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]