Abstract
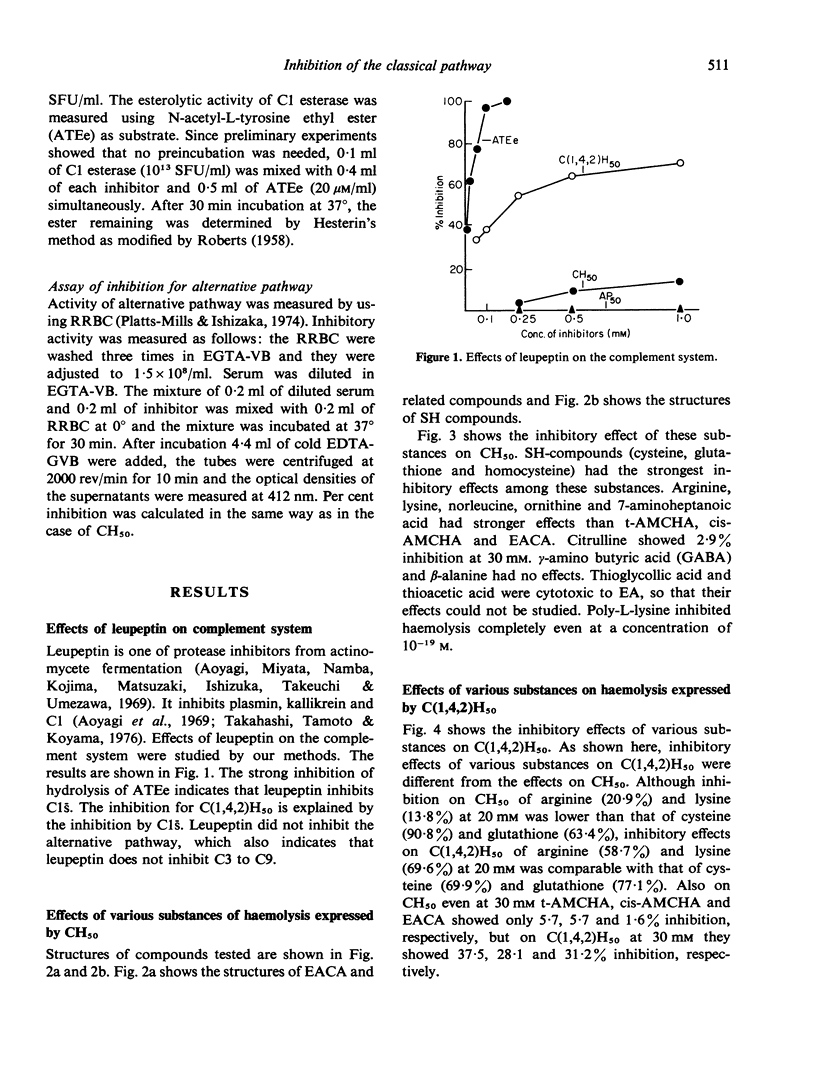
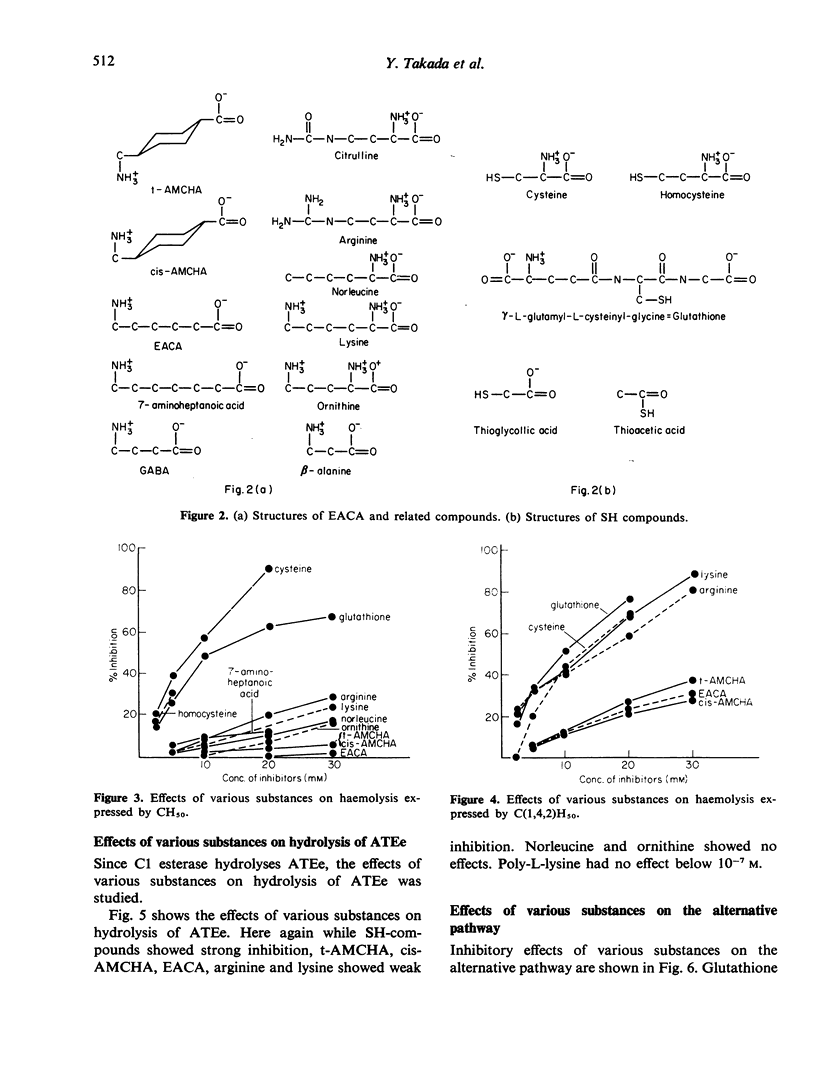
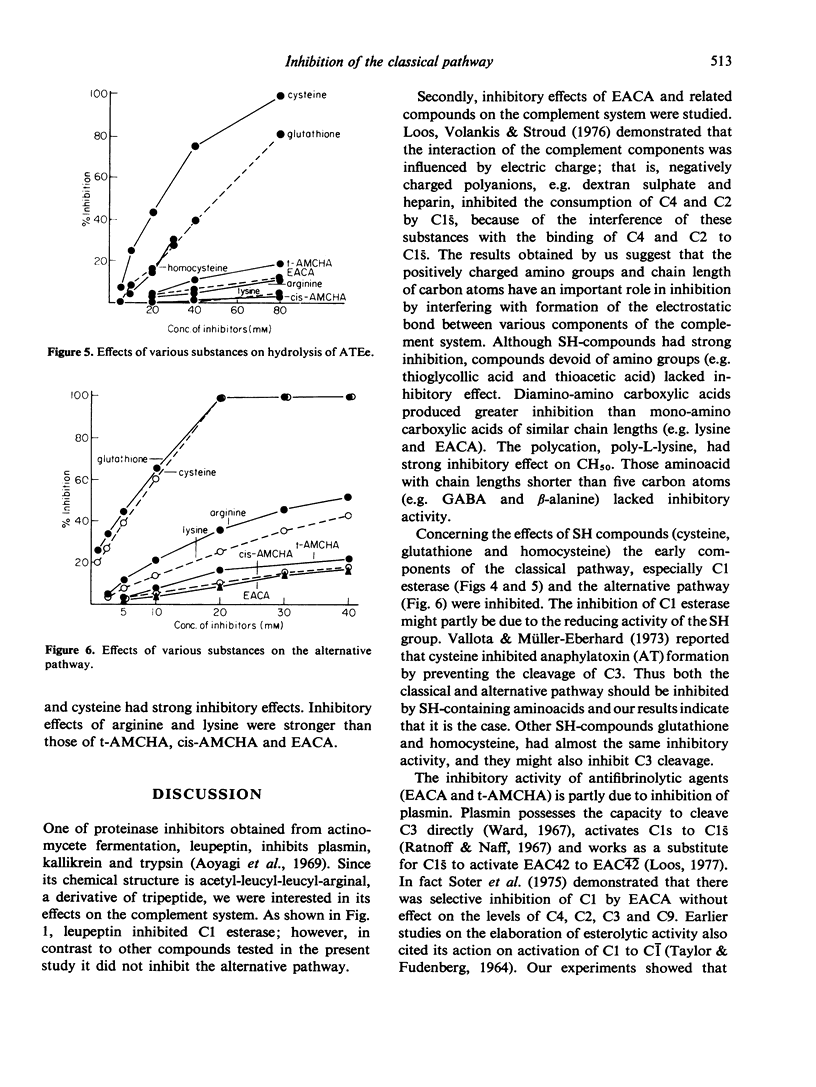
Effects of various aminoacids and their derivatives on the classical pathway and alternative pathway of the complement were studied. Leupeptin, acetyl-leucyl-leucyl-arginal, inhibited CH50 and Cl-esterase, but did not inhibit the alternative pathway. When aminoacids of carbon chains of the order of seven were used, arginine and lysine had stronger effects than trans-aminomethyl cyclohexane carboxylic acid (t-AMCHA), cis-aminomethyl cyclohexane carboxylic acid (cis-AMCHA) and epsilon aminocaproic acid (EACA). SH-compounds, cysteine, homocysteine and glutathione, had the strongest inhibitory effects among these aminoacids on both classical and alternative pathways. When effects on Cl esterase were compared, arginine, lysine, t-AMCHA, cis-AMCHA and EACA had weak inhibition while SH-compounds showed strong inhibition. Poly-L-lysine, which had extremely strong inhibition of CH50, had no inhibition of Cl esterase. The inhibitory effects of antifibrinolytic agents, EACA and t-AMCHA, were weak but when effects on early parts of the classical pathway, C(1,4,2)H50 were tested, some inhibitory activities were recognized. Thus inhibitory effects of these agents were due to their activities on the early parts of the classical pathway.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORSOS T., RAPP H. J. CHROMATOGRAPHIC SEPARATION OF THE FIRST COMPONENT OF COMPLEMENT AND ITS ASSAY ON A MOLECULAR BASIS. J Immunol. 1963 Dec;91:851–858. [PubMed] [Google Scholar]
- Brade V., Nicholson A., Bitter-Suermann D., Hadding U. Formation of the C3-cleaving properdin enzyme on zymosan. Demonstration that factor D is replaceable by proteolytic enzymes. J Immunol. 1974 Dec;113(6):1735–1743. [PubMed] [Google Scholar]
- HAINES A. L., LEPOW I. H. STUDIES ON HUMAN C'1-ESTERASE. I. PURIFICATION AND ENZYMATIC PROPERTIES. J Immunol. 1964 Mar;92:456–467. [PubMed] [Google Scholar]
- Harpel P. C. C1 inactivator inhibition by plasmin. J Clin Invest. 1970 Mar;49(3):568–575. doi: 10.1172/JCI106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M. Plasminogen-plasmin system IX. Specific binding of tranexamic acid to plasmin. Thromb Diath Haemorrh. 1975 Jun 30;33(3):573–585. [PubMed] [Google Scholar]
- Loos M., Volanakis J. E., Stroud R. M. Mode of interaction of different polyanions with the first (C1, C1), the second (C2) and the fourth (C4) component of complement--III. Inhibition of C4 and C2 binding site(s) on C1s by polyanions. Immunochemistry. 1976 Sep;13(9):789–791. doi: 10.1016/0019-2791(76)90202-0. [DOI] [PubMed] [Google Scholar]
- OKAMOTO S., SATO S., TAKADA Y., OKAMOTO U. AN ACTIVE STEREO-ISOMER (TRANS-FORM) OF AMCHA AND ITS ANTIFIBRINOLYTIC (ANTIPLASMINIC) ACTION IN VITRO AND IN VIVO. Keio J Med. 1964 Dec;13:177–185. doi: 10.2302/kjm.13.177. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- ROBERTS P. S. Measurement of the rate of plasmin action on synthetic substrates. J Biol Chem. 1958 May;232(1):285–291. [PubMed] [Google Scholar]
- Ratnoff O. D., Naff G. B. The conversion of C'IS to C'1 esterase by plasmin and trypsin. J Exp Med. 1967 Feb 1;125(2):337–358. doi: 10.1084/jem.125.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soter N. A., Austen K. F., Gigli I. Inhibition by epsilon-aminocaproic acid of the activation of the first component of the complement system. J Immunol. 1975 Mar;114(3):928–932. [PubMed] [Google Scholar]
- TAYLOR F. B., Jr, FUDENBERG H. INHIBITION OF THE C'-1 COMPONENT OF COMPLEMENT BY AMINO ACIDS. Immunology. 1964 Jul;7:319–331. [PMC free article] [PubMed] [Google Scholar]
- Vallota E. H., Müller-Eberhard H. J. Formation of C3a and C5a anaphylatoxins in whole human serum after inhibition of the anaphylatoxin inactivator. J Exp Med. 1973 May 1;137(5):1109–1123. doi: 10.1084/jem.137.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt W., Schmidt G., Lynen R., Dieminger L. Cleavage of the third complement component (C3) and generation of the spasmogenic peptide, C3a, in human serum via the properdin pathway: demonstration of inhibitory as well as enhancing effects of epsilon-amino-caproic acid. J Immunol. 1975 Feb;114(2 Pt 1):671–677. [PubMed] [Google Scholar]
- Ward P. A. A plasmin-split fragment of C'3 as a new chemotactic factor. J Exp Med. 1967 Aug 1;126(2):189–206. doi: 10.1084/jem.126.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]


