Abstract
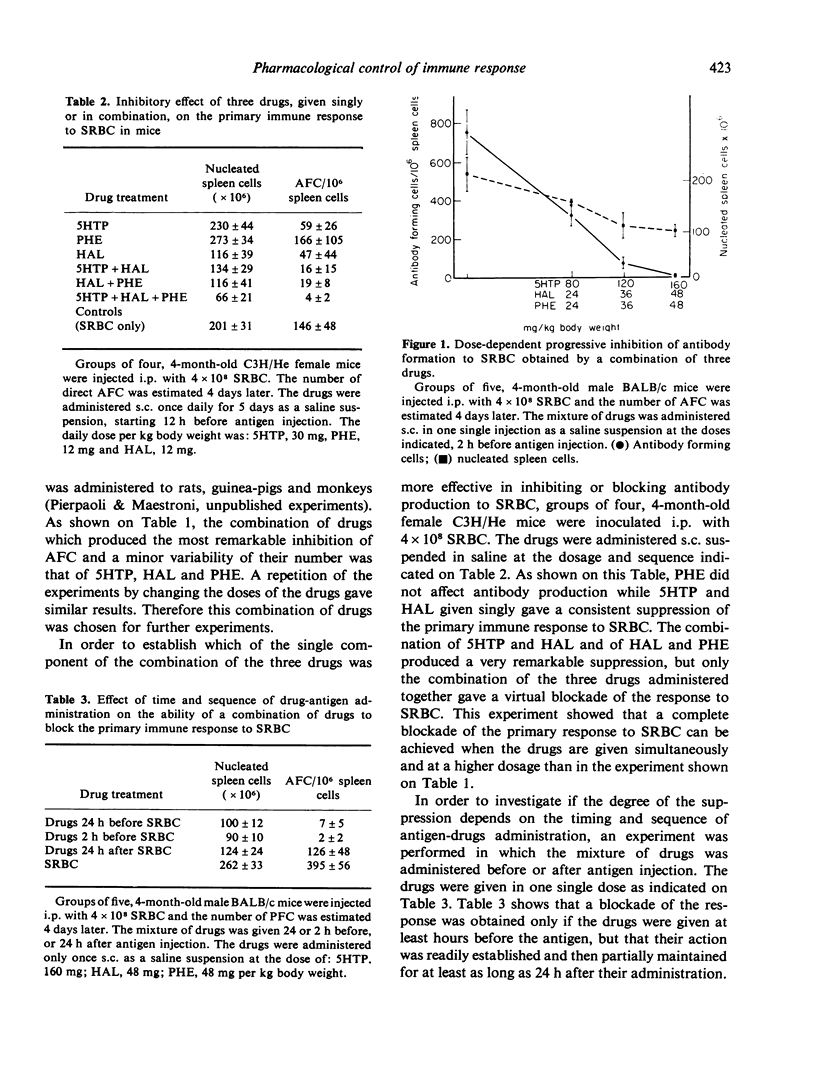
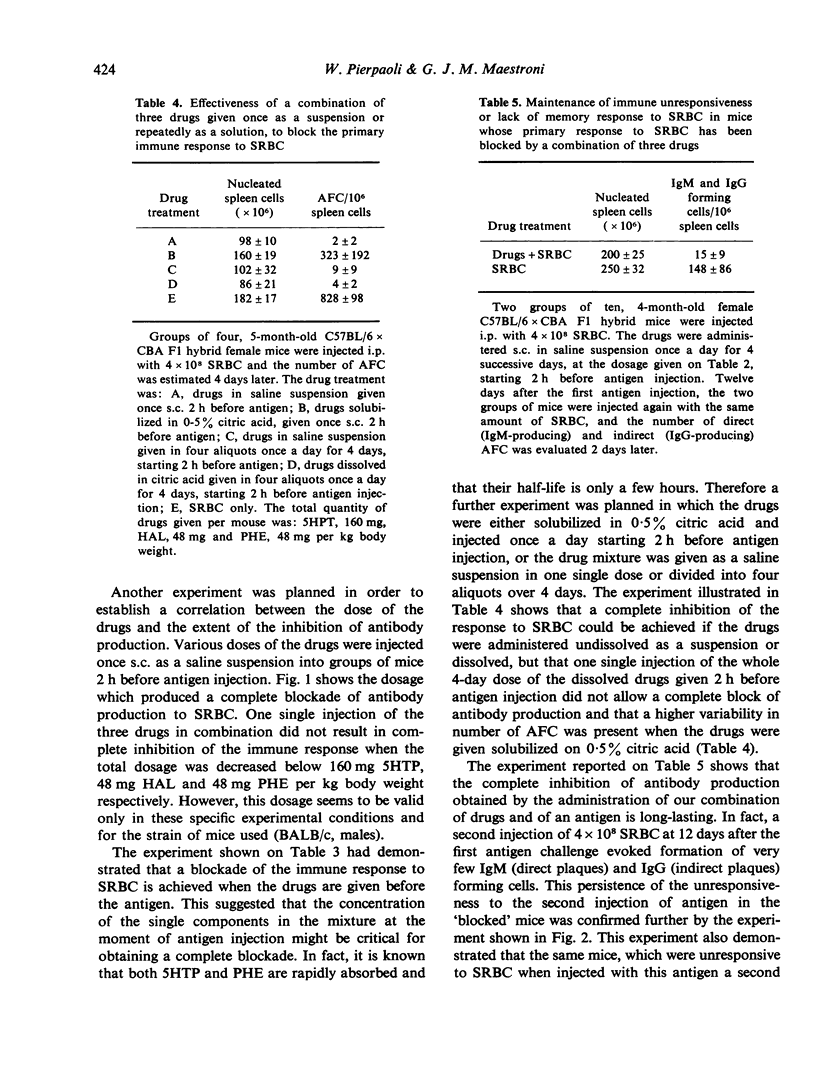
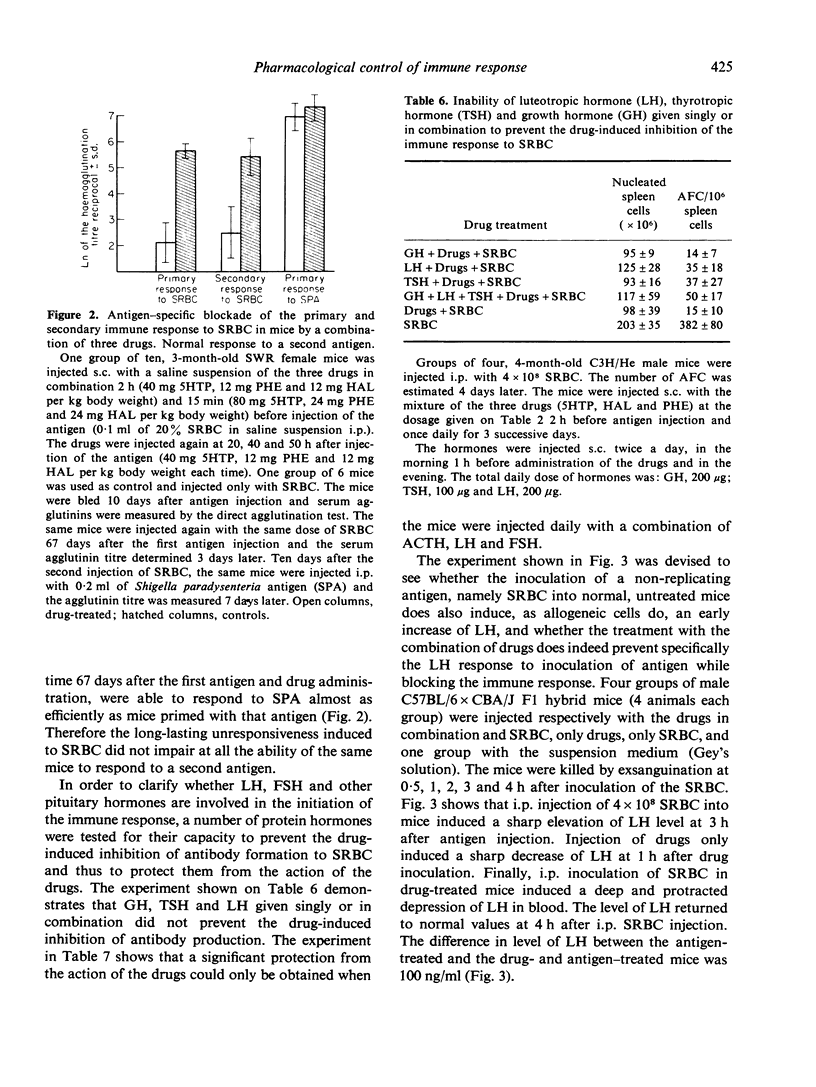
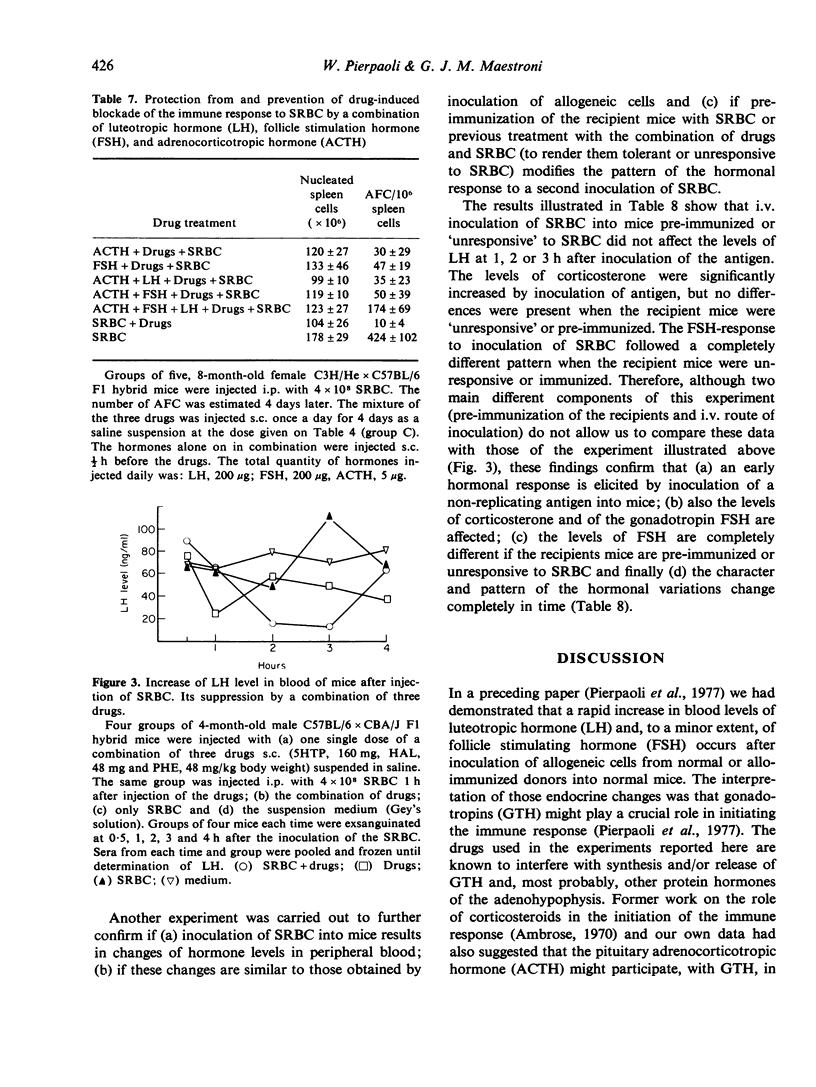
Injection of a combination of three drugs, 5-hydroxytryptophan, the alpha-blocker phentolamine and the neuroleptic drug haloperidol into mice before or together with sheep red blood cells (SRBC) induces a complete and long-lasting inhibition of antibody production to SRBC and leads to specific unresponsiveness. The mice unresponsive to SRBC respond normally to another antigen. Treatment with a combination of luteotropic (LH), follicle stimulating (FSH) and corticotropic hormone (ACTH) before administration of drugs and antigen prevents the immune blockade. Injection of SRBC induces an early elevation of LH in blood. This effect is prevented by previous administration of the three drugs in combination. The hormonal response to a second injection of the same antigen of mice previously made 'unresponsive' is different from that of immunized animals. The suppression of these hormonal changes which follow antigen injection by drugs acting on neuroendocrine regulation and cell membrane adrenergic receptors represents a step forward in efforts aimed at a pharmacological control of acquired immunity.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose C. T. The essential role of corticosteroids in the induction of the immune response in vitro. Ciba Found Study Group. 1970;36:100–125. [PubMed] [Google Scholar]
- Bach M. A. Differences in Cyclic AMP Changes after Stimulation by Prostaglandins and Isoproterenol in Lymphocyte Subpopulations. J Clin Invest. 1975 May;55(5):1074–1081. doi: 10.1172/JCI108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun W., Rega M. J. Adenyl cyclase-stimulating catecholamines as modifiers of antibody formation. Immunol Commun. 1972;1(6):523–532. doi: 10.3109/08820137209022961. [DOI] [PubMed] [Google Scholar]
- Convey E. M., Beal W. E., Seguin B. E., Tannen K. J., Lin Y. C. Gonadotropin releasing hormone induced luteinizing hormone release after prostaglandin F2alpha in heifers. Proc Soc Exp Biol Med. 1976 Jan;151(1):84–88. doi: 10.3181/00379727-151-39148. [DOI] [PubMed] [Google Scholar]
- Deis R. P., Vermouth N. T. Effect of prostaglandin F2 alpha on serum prolactin and lh concentrations in male and female rats. J Reprod Fertil. 1975 Nov;45(2):383–387. doi: 10.1530/jrf.0.0450383. [DOI] [PubMed] [Google Scholar]
- Devoino L., Eliseeva L., Eremina O., Idova G., Cheido M. 5-Hydroxytryptophan effect on the development of the immune response: IgM and IgG antibodies and rosette formation in primary and secondary responses. Eur J Immunol. 1976 Jun;5(6):394–399. doi: 10.1002/eji.1830050608. [DOI] [PubMed] [Google Scholar]
- Franchimont P., Becker H., Ernould C., Thys C., Demoulin A., Bourguignon J. P., Legros J. J., Valcke J. C. The effect of hypothalamic luteinizing hormone releasing hormone (LH-RH) on plasma gonadotrophin levels in normal subjects. Clin Endocrinol (Oxf) 1974 Jan;3(1):27–39. doi: 10.1111/j.1365-2265.1974.tb03293.x. [DOI] [PubMed] [Google Scholar]
- Haran-Ghera N., Peled A. The mechanism of radiation action in leukaemogenesis. Isolation of a leukaemogenic filtrable agent from tissues of irradiated and normal C57BL mice. Br J Cancer. 1967 Dec;21(4):730–738. doi: 10.1038/bjc.1967.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLIMAN B., PETERSON R. E. Double isotope derivative assay of aldosterone in biological extracts. J Biol Chem. 1960 Jun;235:1639–1648. [PubMed] [Google Scholar]
- Lawson D. M., Gala R. R. The influence of adrenergic, dopaminergic, cholinergic and serotoninergic drugs on plasma prolactin levels in ovariectomized, estrogen-treated rats. Endocrinology. 1975 Feb;96(2):313–318. doi: 10.1210/endo-96-2-313. [DOI] [PubMed] [Google Scholar]
- MUELLER J. ALDOSTERONE STIMULATION IN VITRO. I. EVALUATION OF ASSAY PROCEDURE AND DETERMINATION OF ALDOSTERONE-STIMULATING ACTIVITY IN A HUMAN URINE EXTRACT. Acta Endocrinol (Copenh) 1965 Feb;48:283–296. [PubMed] [Google Scholar]
- Man P. L. Long-term effects of haloperidol. Dis Nerv Syst. 1973 Feb;34(2):113–118. [PubMed] [Google Scholar]
- Mertin J., Hunt R. Influence of polyunsaturated fatty acids on survival of skin allografts and tumor incidence in mice. Proc Natl Acad Sci U S A. 1976 Mar;73(3):928–931. doi: 10.1073/pnas.73.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mess B., Péter L. Effect of intracerebral serotonin administration on pituitary-thyroid function. Endocrinol Exp. 1975 Jun;9(2):105–113. [PubMed] [Google Scholar]
- Mortimer C. H., Besser G. M., Hook J., McNeilly A. S. Intravenous, intramuscular, subcutaneous and intranasal administration of LH-FSH-RH: the duration of effect and occurrence of asynchronous pulsatile release of LH and FSH. Clin Endocrinol (Oxf) 1974 Jan;3(1):19–25. doi: 10.1111/j.1365-2265.1974.tb03292.x. [DOI] [PubMed] [Google Scholar]
- Oberst F. W., Crook J. W. Behavioral, physical, and pharmacodynamic effects of Haloperidol in dogs and monkeys. Arch Int Pharmacodyn Ther. 1967 Jun;167(2):450–464. [PubMed] [Google Scholar]
- Pierpaoli W., Haran-Ghera N., Bianchi E., Müller J., Meshorer A., Bree M. Endocrine disorders as a contributory factor to neoplasia in SJL-J mice. J Natl Cancer Inst. 1974 Sep;53(3):731–744. doi: 10.1093/jnci/53.3.731. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W., Haran-Ghera N., Kopp H. G. Role of host endocrine status in murine leukaemogenesis. Br J Cancer. 1977 May;35(5):621–629. doi: 10.1038/bjc.1977.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W., Maestroni G. J. Pharmacological control of the immune response by blockade of the early hormonal changes following antigen injection. Cell Immunol. 1977 Jun 15;31(2):355–363. doi: 10.1016/0008-8749(77)90037-5. [DOI] [PubMed] [Google Scholar]
- Plescia O. J., Yamamoto I., Shimamura T. Cyclic AMP and immune responses: changes in the splenic level of cyclic AMP during the response of mice to antigen. Proc Natl Acad Sci U S A. 1975 Mar;72(3):888–891. doi: 10.1073/pnas.72.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebar R., Yen S. S., VandenBerg G., Naftolin F., Ehara Y., Engblom S., Ryan K. J., Amoss M., Guillemin R. Gonadotropin responses to synthetic LRF: dose-response relationship in men. J Clin Endocrinol Metab. 1973 Jan;36(1):10–16. doi: 10.1210/jcem-36-1-10. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E., Gallager D. A., Sulser F. On the mechanism of brain 5-hydroxytryptamine depletion by p-chloroamphetamine and related drugs and the specificity of their action. Adv Biochem Psychopharmacol. 1974;10:185–194. [PubMed] [Google Scholar]
- Singh U., Owen J. J. Studies on the maturation of thymus stem cells. The effects of catecholamines, histamine and peptide hormones on the expression of T cell alloantigens. Eur J Immunol. 1976 Jan;6(1):59–62. doi: 10.1002/eji.1830060113. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Osheroff P. L. Antigen stimulation of prostaglandin synthesis and control of immune responses. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1300–1304. doi: 10.1073/pnas.73.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto I., Webb D. R. Antigen-stimulated changes in cyclic nucleotide levels in the mouse. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2320–2324. doi: 10.1073/pnas.72.6.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]


