Abstract
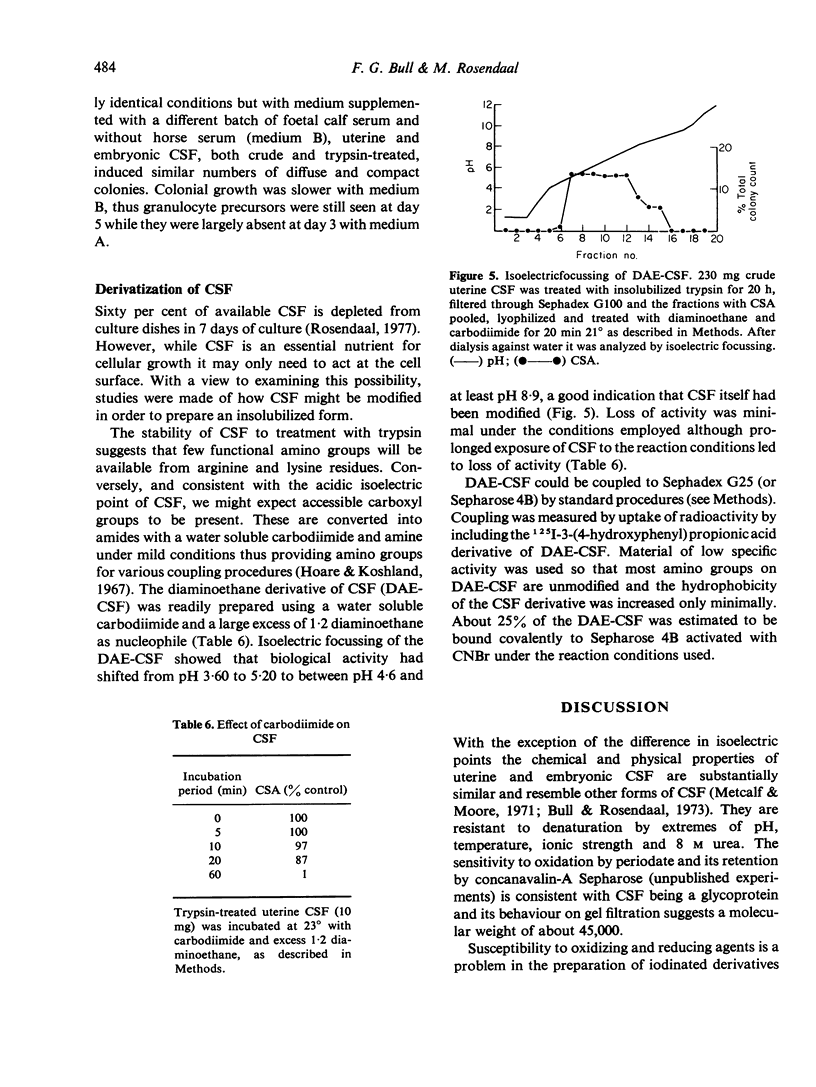
Extracts from embryonic and uterine tissue of mice, operationally defined as colony stimulating factor (CSF), promoted the growth of macrophage-granulocyte colonies in vitro. Uterine CSF focusses from pH 5.15 to 6.00 and embryonic CSF from pH 3.60 to 5.20, although both forms have similar biological activity. CSF is relatively resistant to denaturation but it is inactivated by periodate and dithiothreitol. Gel filtration indicates a molecular weight of 45,000 which is unchanged following treatment with insolubilized trypsin, a procedure which affords a useful purification (240-fold). Trypsin-sensitive material in CSF preparations modifies colonial form under certain conditions of culture, probably by increasing the motility of macrophages. Diaminoethane derivatives of CSF were prepared and retained biological activity at isoelectric points above pH 9.0. These derivatives may be covalently linked to Sepharose providing an insolubilized form of CSF to study interactions of CSF with the cell surface.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin P. E., McCulloch E. A., Till J. E. Characterization of the factor in L-cell conditioned medium capable of stimulating colony formation by mouse marrow cells in culture. J Cell Physiol. 1971 Apr;77(2):121–134. doi: 10.1002/jcp.1040770202. [DOI] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Stanley E. R., Sumner M. A. Factors from mouse tissues stimulating colony growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1971 Dec;49(6):595–603. doi: 10.1038/icb.1971.65. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Roitt I. M. The effect of phytohaemagglutinin and other lymphocyte mitogens on immunoglobulin synthesis by human peripheral blood lymphocytes in vitro. Clin Exp Immunol. 1968 Jun;3(5):393–412. [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Metcalf D. Studies on colony formation in vitro by mouse bone marrow cells. I. Continuous cluster formation and relation of clusters to colonies. J Cell Physiol. 1969 Dec;74(3):323–332. doi: 10.1002/jcp.1040740313. [DOI] [PubMed] [Google Scholar]
- Revoltella R., Bertolini L., Pediconi M., Vigneti E. Specific binding of nerve growth factor (NGF) by murine C 1300 neuroblastoma cells. J Exp Med. 1974 Aug 1;140(2):437–451. doi: 10.1084/jem.140.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendaal M. An estimate of the extent of depletion of colony-stimulating factors in culture. Exp Hematol. 1977 Mar;5(2):149–152. [PubMed] [Google Scholar]
- Rosendaal M. Colony-stimulating factor (CSF) in the uterus of the pregnant mouse. J Cell Sci. 1975 Nov;19(2):411–423. doi: 10.1242/jcs.19.2.411. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Metcalf D. Enzyme treatment of colony stimulating factor: evidence for a peptide component. Aust J Exp Biol Med Sci. 1971 Jun;49(3):281–290. doi: 10.1038/icb.1971.28. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Metcalf D. Partial purification and some properties of the factor in normal and leukaemic human urine stimulating mouse bone marrow colony growth in vitro. Aust J Exp Biol Med Sci. 1969 Aug;47(4):467–483. doi: 10.1038/icb.1969.51. [DOI] [PubMed] [Google Scholar]
- Trotta P. P., Pinkus L. M., Meister A. Inhibition by dithiothreitol of the utilization of glutamine by carbamyl phosphate synthetase. Evidence for formation of hydrogen peroxide. J Biol Chem. 1974 Mar 25;249(6):1915–1921. [PubMed] [Google Scholar]