Abstract
A comparison of the in vivo and in vitro antibody response capabilities of two marmoset species, Saguinus fuscicollis and Saguinus oedipus oedipus, revealed the former to be superior in elaborating humoral antibody. In vivo challenges with Escherichia coli lipopolysaccharide (LPS) and Salmonella typhi flagella consistently yielded higher antibody titres in S. fuscicollis; indeed, with LPS antigen, multiple inoculations of S.o. oedipus marmosets led ultimately to a decrease in antibody formation, in contrast to the anamnestic response of S. fuscicollis. This species differential in immune competence was also suggested in the in vitro stimulation of peripheral blood leucocytes (PBL) and spleen cells with sheep red blood cells (RBC). None of 55 S.o. oedipus PBL cultures and 49 of 89 (55%) S. fuscicollis cultures responded to the test antigen. A similar differential in response to sheep RBC was noted with the spleen cells of each species, although this report contrasts the antibody-forming potential of two marmoset species, a comparison of the immunological response profile of marmosets to those of other laboratory animals challenged with similar antigens suggests these primates may be relatively incompetent. The possible relationship between the haemopoietic chimerism of marmosets and a diminished immune competence is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Barnhart D. D., Gengozian N. An evaluation of the mixed lymphocyte culture reaction in marmosets. Transplantation. 1975 Aug;20(2):107–115. doi: 10.1097/00007890-197508000-00003. [DOI] [PubMed] [Google Scholar]
- Bechtol K. B., McDevitt H. O. Antibody response of C3H in equilibrium (CKB X CWB)F1 tetraparental mice to poly-L(Tyr,Glu)-poly-D,L-Ala-poly-L-Lys immunization. J Exp Med. 1976 Jul 1;144(1):123–144. doi: 10.1084/jem.144.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtol K. B., Wegmann T. G., Freed J. H., Grumet F. C., Chesebro B. W., Herzenberg L. A., McDevitt H. O. Genetic control of the immune response to (T,G)-A--L in C3H in equilibrium C57 tetraparental mice. Cell Immunol. 1974 Aug;13(2):264–277. doi: 10.1016/0008-8749(74)90244-5. [DOI] [PubMed] [Google Scholar]
- Benirschke K., Anderson J. M., Brownhill L. E. Marrow Chimerism in Marmosets. Science. 1962 Oct 26;138(3539):513–515. doi: 10.1126/science.138.3539.513. [DOI] [PubMed] [Google Scholar]
- Britton S. Regulation of antibody synthesis against Escherichia coli endotoxin. II. Specificity, dose requirements and duration of paralysis induced in adult mice. Immunology. 1969 Apr;16(4):513–526. [PMC free article] [PubMed] [Google Scholar]
- Cosenza H., Leserman L. D., Rowley D. A. The third cell type required for the immune response of spleen cells in vitro. J Immunol. 1971 Aug;107(2):414–421. [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V., Dietrich F. M. The morphology of immune reactions in normal, thymectomized and reconstituted mice. 3. Response to bacterial antigens: salmonellar flagellar antigen and pneumococcal plysaccharide. Immunology. 1970 Dec;19(6):945–957. [PMC free article] [PubMed] [Google Scholar]
- Deinhardt F., Wolfe L., Northrop R., Marczynska B., Ogden J., McDonald R., Falk L., Shramek G., Smith R., Deinhardt J. Induction of neoplasms by viruses in marmoset monkeys. J Med Primatol. 1972;1(1):29–50. doi: 10.1159/000460360. [DOI] [PubMed] [Google Scholar]
- Delfraissy J. F., Galanaud P., Dormont J., Wallon C. Primary in vitro antibody response from human peripheral blood lymphocytes. J Immunol. 1977 Feb;118(2):630–635. [PubMed] [Google Scholar]
- Dosch H-M, Gelfand E. W. Generation of human plaque-forming cells in culture: tissue distribution, antigenic and cellular requirements. J Immunol. 1977 Jan;118(1):302–308. [PubMed] [Google Scholar]
- Friedman H., Allen J., Rosenzweig J. Comparison of bacteriolysis, passive hemolysis, and bacterial adherence colony formation for detecting antibody-forming cells. Proc Soc Exp Biol Med. 1969 Jun;131(2):353–359. doi: 10.3181/00379727-131-33876. [DOI] [PubMed] [Google Scholar]
- GENGOZIAN N., BATSON J. S., EIDE P. HEMATOLOGIC AND CYTOGENETIC EVIDENCE FOR HEMATOPOIETIC CHIMERISM IN THE MARMOSET, TAMARINUS NIGRICOLLIS. Cytogenetics. 1964;3:384–393. doi: 10.1159/000129828. [DOI] [PubMed] [Google Scholar]
- Gengozian N., Batson J. S., Greene C. T., Gosslee D. G. Hemopoietic chimerism in imported and laboratory-bred marmosets. Transplantation. 1969 Nov;8(5):633–652. doi: 10.1097/00007890-196911000-00009. [DOI] [PubMed] [Google Scholar]
- Gengozian N., Batson J. S., Smith T. A. Breeding of marmosets in a colony environment. Primates Med. 1978;10:71–78. [PubMed] [Google Scholar]
- Gengozian N. Marmosets: their potential in experimental medicine. Ann N Y Acad Sci. 1969 Jul 3;162(1):336–362. doi: 10.1111/j.1749-6632.1969.tb56381.x. [DOI] [PubMed] [Google Scholar]
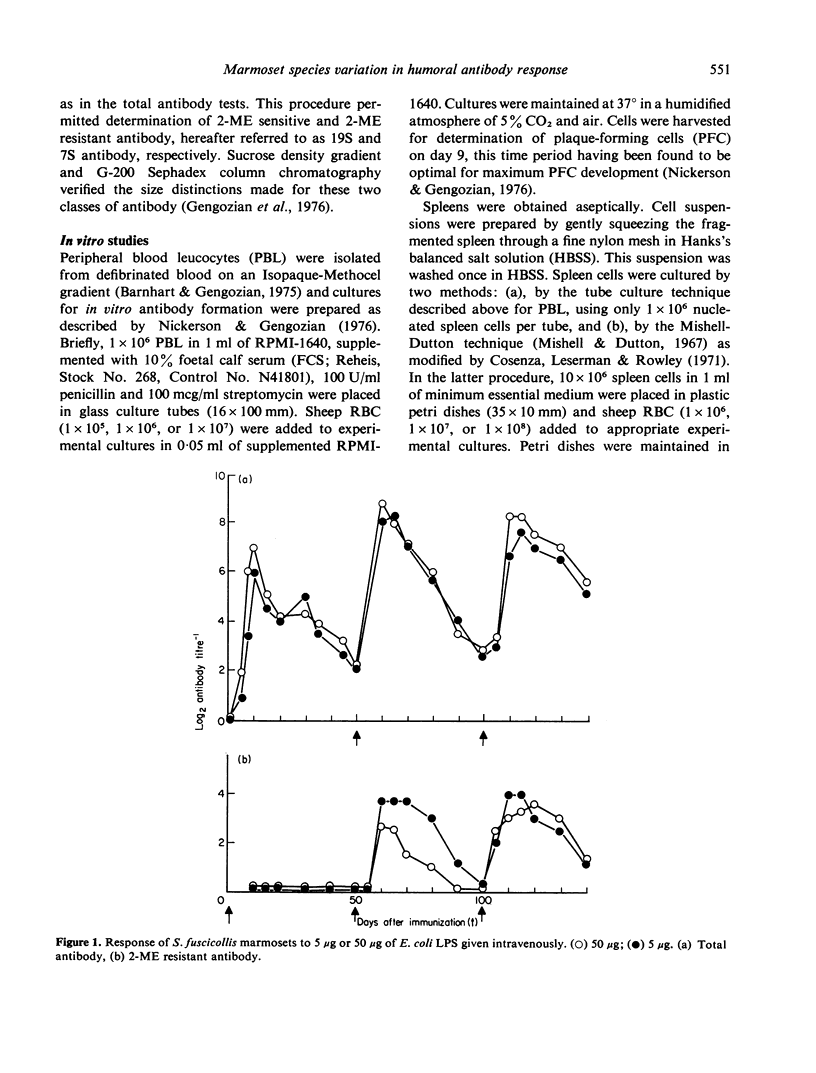
- Gengozian N., Salter B. L., Basford N. L., Kateley J. R. Characterization of the antibody response of the marmoset to sheep red blood cells. Clin Exp Immunol. 1976 Mar;23(3):525–535. [PMC free article] [PubMed] [Google Scholar]
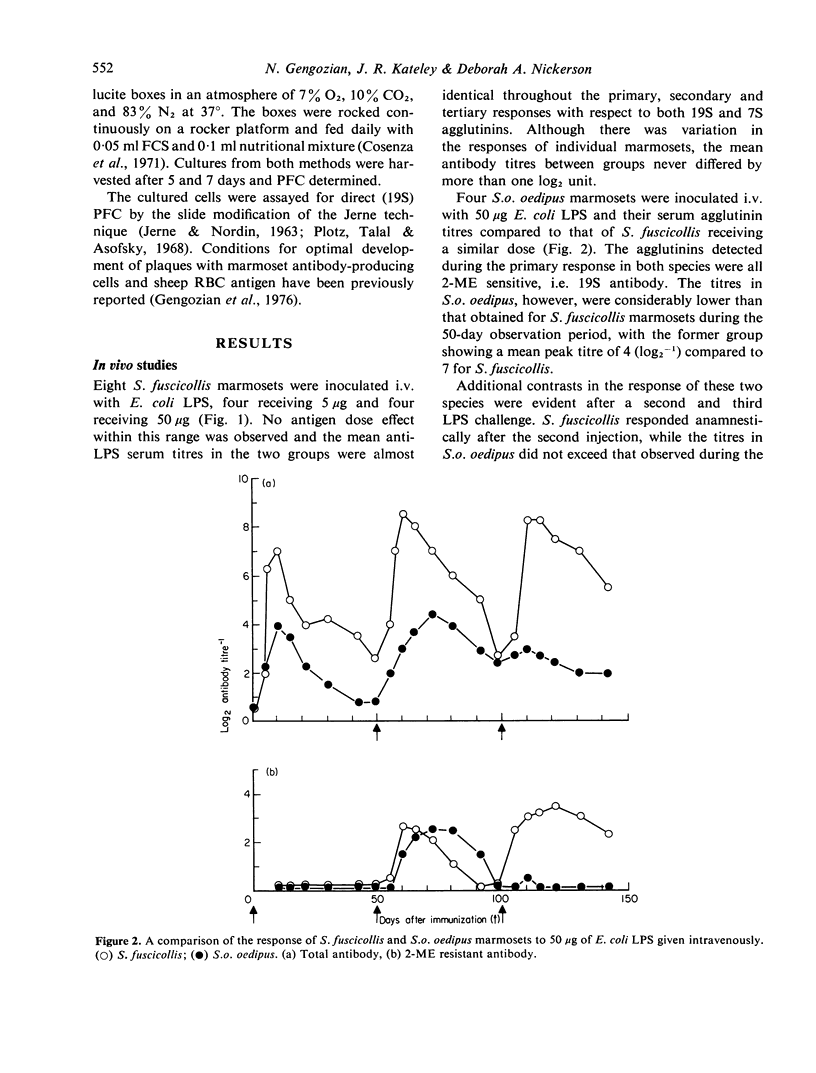
- HUMPHREY J. H., PARROTT D. M., EAST J. STUDIES ON GLOBULIN AND ANTIBODY PRODUCTION IN MICE THYMECTOMIZED AT BIRTH. Immunology. 1964 Jul;7:419–439. [PMC free article] [PubMed] [Google Scholar]
- Harvey J. S., Jr, Felsburg P. J., Heberling R. L., Kniker W. T., Kalter S. S. Immunological competence in non-human primates: differences observed in four species. Clin Exp Immunol. 1974 Feb;16(2):267–277. [PMC free article] [PubMed] [Google Scholar]
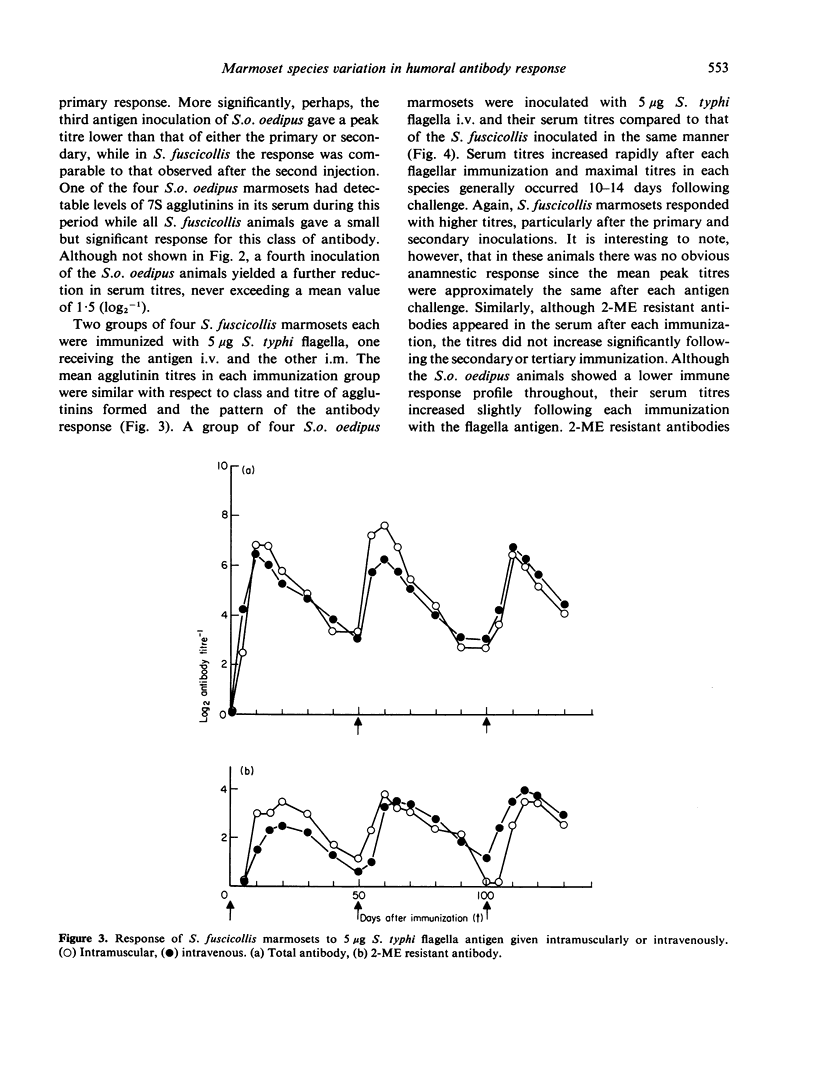
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
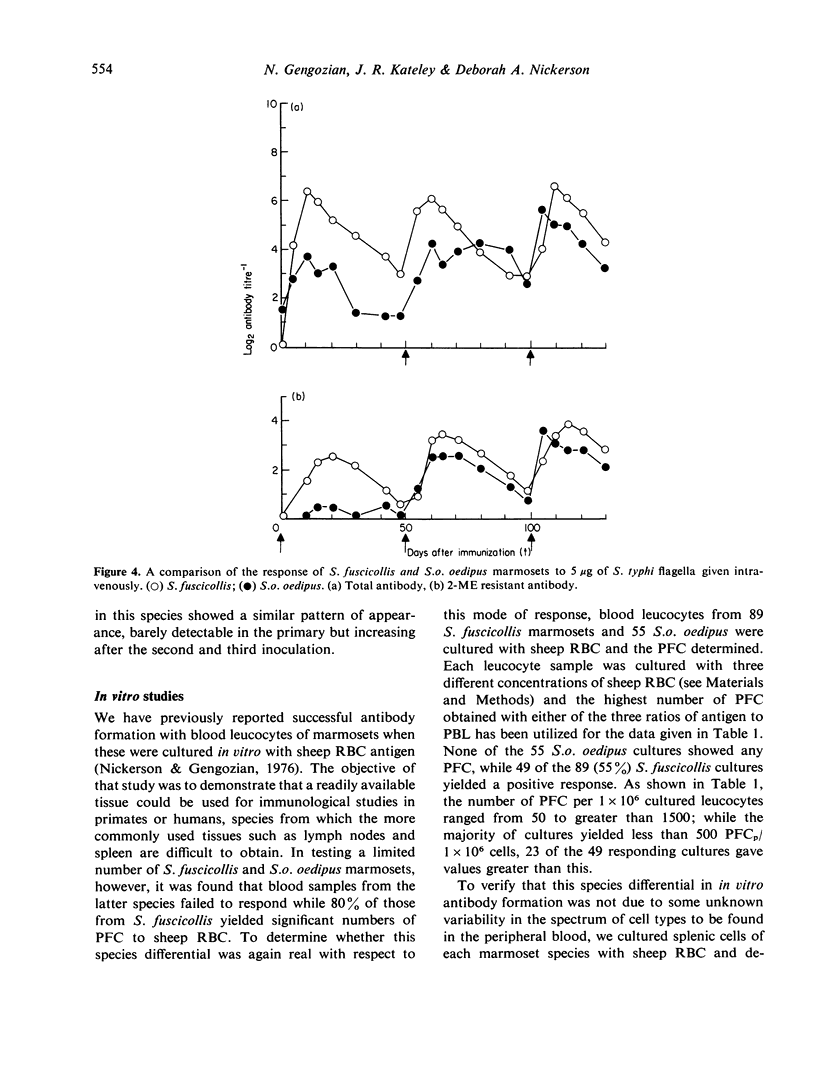
- Jackson A. L., Walters C. S. Cells producing low-molecular-weight antibody to Escherichia coli lipopolysaccharide. Infect Immun. 1972 Oct;6(4):545–549. doi: 10.1128/iai.6.4.545-549.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kateley J. R., Nickerson D. A., Gengozian N. Mitogenic response of marmoset lymphocytes: cytokinetics and identification of responsive cells. Immunology. 1977 Nov;33(5):653–662. [PMC free article] [PubMed] [Google Scholar]
- Luzzati A. L., Taussig M. J., Meo T., Pernis B. Induction of an antibody response in cultures of human peripheral blood lymphocytes. J Exp Med. 1976 Sep 1;144(3):573–585. doi: 10.1084/jem.144.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G., Michael G. Frequency of antigen-sensitive cells to thymus-independent antigens. Cell Immunol. 1971 Aug;2(4):309–316. doi: 10.1016/0008-8749(71)90065-7. [DOI] [PubMed] [Google Scholar]
- NOSSAL G. J., ADA G. L., AUSTIN C. M. ANTIGENS IN IMMUNITY. II. IMMUNOGENIC PROPERTIES OF FLAGELLA, POLYMERIZED FLAGELLIN AND FLAGELLIN IN THE PRIMARY RESPONSE. Aust J Exp Biol Med Sci. 1964 Jun;42:283–294. [PubMed] [Google Scholar]
- Niblack G. D., Gengozian N. T and B lymphocytes in the marmoset: a natural haemopoietic chimera. Clin Exp Immunol. 1976 Mar;23(3):536–543. [PMC free article] [PubMed] [Google Scholar]
- Nickerson D. A., Gengozian N. Primary in vitro antibody formation by blood leucocytes of a subhuman primate. Cell Immunol. 1976 Dec;27(2):171–176. doi: 10.1016/0008-8749(76)90226-4. [DOI] [PubMed] [Google Scholar]
- Plotz P. H., Talal N., Asofsky R. Assignment of direct and facilitated hemolytic plaques in mice to specific immunoglobulin classes. J Immunol. 1968 Apr;100(4):744–751. [PubMed] [Google Scholar]
- Porter R. P., Gengozian N. Immunological responsiveness and tolerance of marmoset lymphoid tissue in vitro. Transplantation. 1973 Feb;15(2):221–230. doi: 10.1097/00007890-197302000-00006. [DOI] [PubMed] [Google Scholar]
- Porter R. P., Gengozian N. Immunological tolerance and rejection of skin allografts in hhe marmoset. Transplantation. 1969 Nov;8(5):653–665. doi: 10.1097/00007890-196911000-00010. [DOI] [PubMed] [Google Scholar]
- Rowley M. J., Mackay I. R. Measurement of antibody-producing capacity in man. I. The normal response to flagellin from Salmonella adelaide. Clin Exp Immunol. 1969 Oct;5(4):407–418. [PMC free article] [PubMed] [Google Scholar]
- Shinohara N., Kern M. Differentiation of lymphoid cells: B cell as a direct target and T cell as a regulator in lipopolysaccharide-enhanced induction of immunoglobulin production. J Immunol. 1976 Jun;116(6):1607–1612. [PubMed] [Google Scholar]
- Urso P., Gengozian N. T cell deficiency in mouse allogeneic radiation chimeras. J Immunol. 1973 Sep;111(3):712–719. [PubMed] [Google Scholar]
- Urso P., Gengozian N. Variation in T and B cell deficiency in different mouse allogeneic radiation chimeras. J Immunol. 1974 Dec;113(6):1770–1779. [PubMed] [Google Scholar]
- Warner C. M., Fitzmaurice M., Maurer P. H., Merryman C. F., Schmerr M. J. The immune response of tetraparental mice to two synthetic amino acid polymers: "high-conjugation" 2,4 dinitrophenyl-glutamic acid57-lysine38-alanine5 (DNP-GLA5) and glutamic acid60 alanine30 tyrosine10 (GAT10). J Immunol. 1973 Dec;111(6):1887–1893. [PubMed] [Google Scholar]


