Abstract
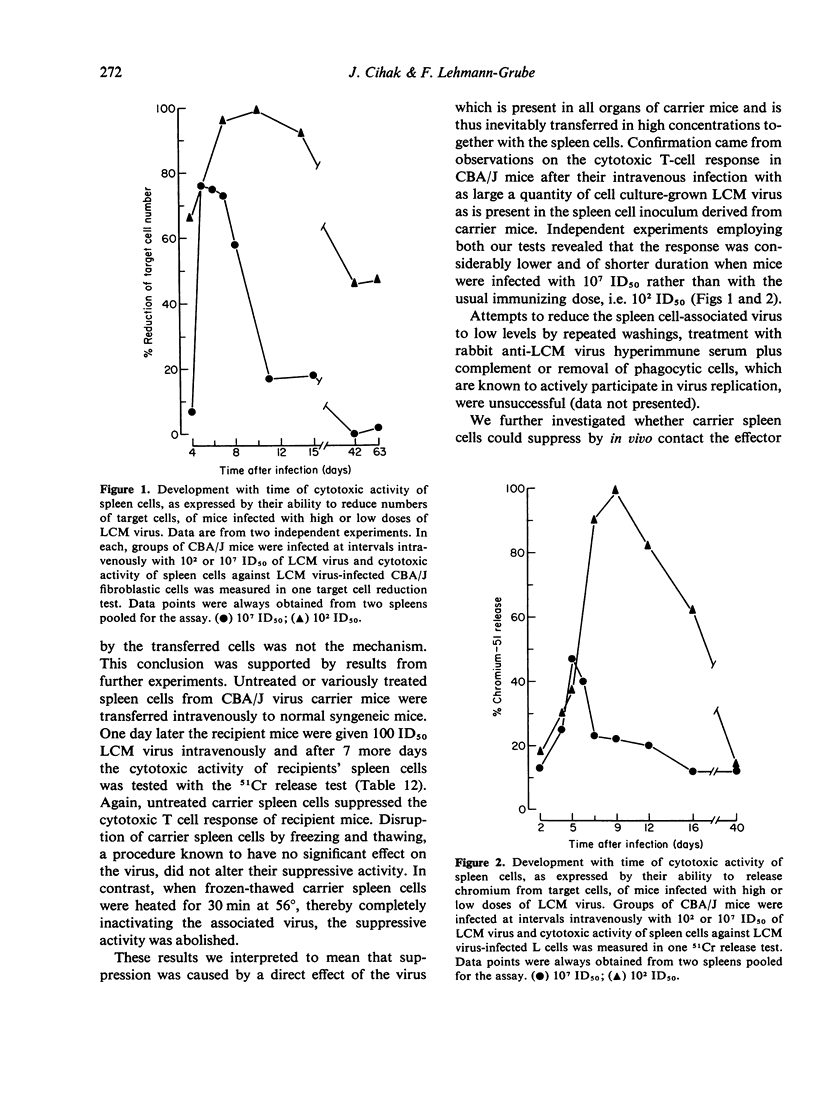
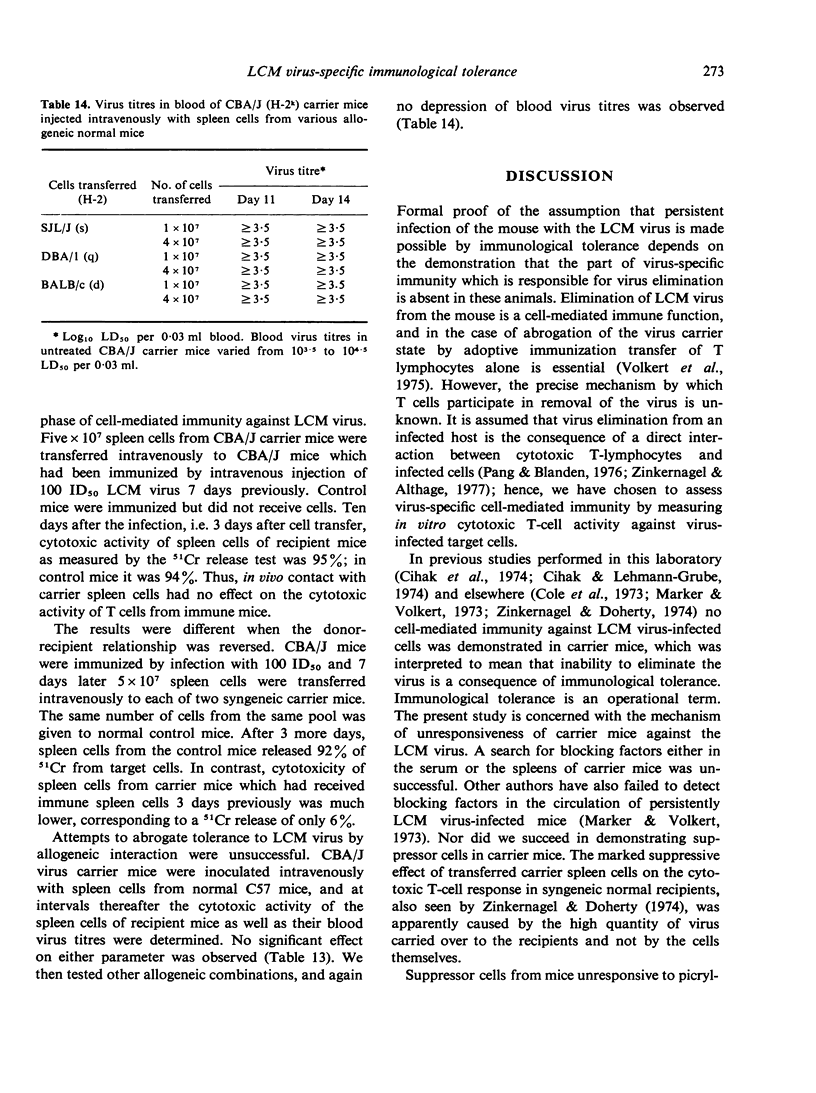
Previous studies have shown that no cell-mediated immunity against LCM virus-infected cells can be detected in neonatally established LCM virus carrier mice suggesting that they are immunologically tolerant to virally-altered cell membrane antigens. In this communication experiments are described aimed at analyzing the mechanism. Virus-specific cell-mediated immunity was assessed by 51Cr release and target cell reduction assays. Attempts to demonstrate cells in spleens of CBA/J carrier mice able to suppress in syngeneic recipients the induction or the effector phase of the cytotoxic T-cell response against LCM virus-infected cells were unsuccessful. Also, no factors were detected in CBA/J and C57BL/6J carrier mice, either spleen cell-associated or free in the circulation, which would block the activity of cytotoxic T-lymphocytes against LCM virus-infected syngeneic target cells. The results indicate that inability of LCM virus carrier mice to act immunologically against virus-infected target cells is due to deletion or irreversible inactivation of T lymphocytes carrying receptors for virally altered cell membrane antigens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basten A., Miller J. F., Sprent J., Cheers C. Cell-to-cell interaction in the immune response. X. T-cell-dependent suppression in tolerant mice. J Exp Med. 1974 Jul 1;140(1):199–217. doi: 10.1084/jem.140.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson L., Hotchin J. Antibody formation in persistent tolerant infection with lymphocytic choriomeningitis virus. Nature. 1969 Jun 14;222(5198):1045–1047. doi: 10.1038/2221045a0. [DOI] [PubMed] [Google Scholar]
- Boehmer H., Sprent J., Nabholz M. Tolerance to histocompatibility determinants in tetraparental bone marrow chimeras. J Exp Med. 1975 Feb 1;141(2):322–334. doi: 10.1084/jem.141.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C. G. Neonatally induced transplantation tolerance: in vitro evidence supporting a clonal inactivation mechanism. Eur J Immunol. 1975 Nov;5(11):741–747. doi: 10.1002/eji.1830051103. [DOI] [PubMed] [Google Scholar]
- Cihak J., Lehmann-Grube F., Cihak J. Immunphänomene bei LCM-virusinfizierten Mäusen. Zentralbl Bakteriol Orig A. 1974;227(1-4):450–457. [PubMed] [Google Scholar]
- Cihak J., Lehmann-Grube F. Persistent infection of mice with the virus of lymphocytic choriomeningitis: virus-specific immunological tolerance. Infect Immun. 1974 Nov;10(5):1072–1076. doi: 10.1128/iai.10.5.1072-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M., Ramshaw I. A. Specificity and development of cytotoxic thymus-derived lymphocytes in lymphocytic choriomeningitis. J Immunol. 1974 Apr;112(4):1548–1552. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Infectious immunological tolerance. Immunology. 1971 Dec;21(6):903–914. [PMC free article] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Lymphocyte-mediated cytotoxicity and blocking serum activity to tumor antigens. Adv Immunol. 1974;18:209–277. doi: 10.1016/s0065-2776(08)60311-9. [DOI] [PubMed] [Google Scholar]
- Kölsch E., Stumpf R., Weber G. Low zone tolerance and suppressor T cells. Transplant Rev. 1975;26:56–86. doi: 10.1111/j.1600-065x.1975.tb00175.x. [DOI] [PubMed] [Google Scholar]
- LEHMANN-GRUBE F. LYMPHOCYTIC CHORIOMENINGITIS IN THE MOUSE. I. GROWTH IN THE BRAIN. Arch Gesamte Virusforsch. 1964;14:344–350. doi: 10.1007/BF01555827. [DOI] [PubMed] [Google Scholar]
- LEHMANN-GRUBE F. LYMPHOCYTIC CHORIOMENINGITIS IN THE MOUSE. II. ESTABLISHMENT OF CARRIER COLONIES. Arch Gesamte Virusforsch. 1964;14:351–357. doi: 10.1007/BF01555828. [DOI] [PubMed] [Google Scholar]
- LOCKART R. Z., Jr, EAGLE H. Requirements for growth of single human cells. Science. 1959 Jan 30;129(3344):252–254. doi: 10.1126/science.129.3344.252. [DOI] [PubMed] [Google Scholar]
- Marker O., Volkert M. Studies on cell-mediated immunity to lymphocytic choriomeningitis virus in mice. J Exp Med. 1973 Jun 1;137(6):1511–1525. doi: 10.1084/jem.137.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P. J. The abrogation of sheep erythrocyte tolerance in rats by means of the transfer of allogeneic lymphocytes. J Exp Med. 1970 Nov;132(5):916–925. doi: 10.1084/jem.132.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Chiller J. M., Weigle W. O., Dixon F. J. Effect of chronic viral infection on the immune system. I. Comparison of the immune responsiveness of mice chronically infected with LCM virus with that of noninfected mice. J Immunol. 1973 May;110(5):1268–1278. [PubMed] [Google Scholar]
- Pang T., Blanden R. V. Regulation of the T-cell response to ectromelia virus infection. I. Feedback suppression by effector T cells. J Exp Med. 1976 Mar 1;143(3):469–481. doi: 10.1084/jem.143.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A. E., Allen J. M. Mouse thymic iso-antigens. Nature. 1966 Jan 29;209(5022):521–523. doi: 10.1038/209521b0. [DOI] [PubMed] [Google Scholar]
- Silvers W. K., Elkins W. L., Quimby F. W. Cellular basis of tolerance in neonatally induced mouse chimeras. J Exp Med. 1975 Nov 1;142(5):1312–1315. doi: 10.1084/jem.142.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLKERT M., LARSEN J. H., PFAU C. STUDIES ON IMMUNOLOGICAL TOLERANCE TO LCM VIRUS. 4. THE QUESTION OF IMMUNITY IN ADOPTIVELY IMMUNIZED VIRUS CARRIERS. Acta Pathol Microbiol Scand. 1964;61:268–282. doi: 10.1111/apm.1964.61.2.268. [DOI] [PubMed] [Google Scholar]
- Volkert M., Bro-Jorgensen K., Marker O., Rubin B., Trier L. The activity of T and B lymphocytes in immunity and tolerance to the lymphocytic choriomeningitis virus in mice. Immunology. 1975 Sep;29(3):455–464. [PMC free article] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L. Depression of the T cell phenomenon of contact sensitivity by T cells from unresponsive mice. Nature. 1973 Jul 27;244(5413):227–228. doi: 10.1038/244227a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A. Antiviral protection by virus-immune cytotoxic T cells: infected target cells are lysed before infectious virus progeny is assembled. J Exp Med. 1977 Mar 1;145(3):644–651. doi: 10.1084/jem.145.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. H-2 compatability requirement for T-cell-mediated lysis of target cells infected with lymphocytic choriomeningitis virus. Different cytotoxic T-cell specificities are associated with structures coded for in H-2K or H-2D;. J Exp Med. 1975 Jun 1;141(6):1427–1436. doi: 10.1084/jem.141.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]


