Abstract
Objective To review the clinical presentations and diagnostic issues in adrenomyeloneuropathy and adrenoleukodystrophy, which are different presentations of the same single gene disorder.
Design Observational study.
Participants Three generations of an affected kindred.
Intervention None.
Main outcome measures Neurological features suggestive of adrenoleukodystrophy or adrenomyeloneuropathy. Measurement of very long chain fatty acids. Molecular analysis of the adrenoleukodystrophy gene.
Results Three adults presented with adrenomyeloneuropathy and two children with adrenoleukodystrophy. Circulating concentrations of long chain fatty acids were raised consistent with clinical features. A mutation in exon 6 of the adrenoleukodystrophy gene (P543L) was identified. This had not previously been identified but has subsequently been reported by other groups.
Conclusions Adrenomyeloneuropathy should be considered in the differential diagnosis in male patients presenting with adrenal failure. Early diagnosis allows genetic counselling in such families and may become more important as treatment strategies evolve.
INTRODUCTION
Adrenal failure in adults in the UK is most commonly autoimmune in origin. However, other causes are occasionally seen with early identification having implications for treatment, prognosis and, where appropriate, genetic counselling. This report describes a kindred with adult onset adrenomyeloneuropathy and childhood onset adrenoleukodystrophy due to a single gene mutation. The index case presented with adrenal failure and subsequently developed neurological disease. Adrenomyeloneuropathy was seen in two other adult family members with adrenoleukodystrophy being diagnosed in two children. Adult onset adrenomyeloneuropathy and childhood onset adrenoleukodystrophy occurring in the same family is a recognised phenomenon although the explanation for the diverse clinical presentations in patients with the same mutation remains to be elucidated.1 Adult onset adrenomyeloneuropathy, presenting with primary adrenal failure, can pose diagnostic difficulty.2 The purpose of this report is to consider diagnostic issues and to briefly review what is currently known about the molecular basis of the condition and potential treatments.
METHODS
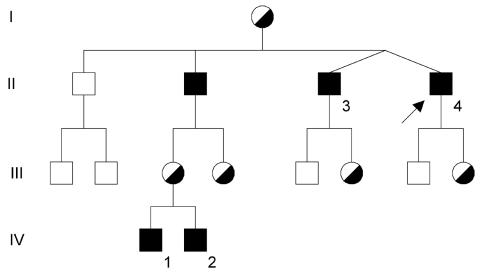
Assessment of case histories: Three generations of an affected kindred were studied (Figure 1).
Figure 1.
Family tree. The index case is marked with an arrow
Biochemistry: Very long chain fatty acids were measured by stable isotope GCMS (gas chromatography mass spectrometry).
Molecular genetic analysis: Leucocyte DNA was extracted by standard methods. SSCP analysis of each of the exons of the adrenoleukodystrophy gene suggested a mutation in exon 6. Direct sequencing of both strands of an exon 6 PCR product was undertaken.
RESULTS
Case descriptions
Index case (Figure 1, case II-4)
A 31-year-old previously asymptomatic man presented in 1992, with a 6-day history of sore throat and 3 days of abdominal pain. He was acutely unwell with a Glasgow Coma Score of 7/15, central cyanosis and unrecordable blood pressure. Investigations revealed a leucocytosis (34.4 × 109/L), hyponatraemia (sodium 120mmol/L) and acute renal impairment (creatinine 614 μmol/L). Plasma potassium was 4.5 mmol/L, glucose 6.3 mmol/L and cortisol 229mmol/L. With intravenous fluids, hydrocortisone and antibiotics he made a full recovery. A short synacthen test confirmed adrenal failure with a baseline cortisol of 17 nmol/L and 30 nmol/L at 30 min. Adrenal antibodies were absent and abdominal computerized tomography scan showed only atrophied adrenal glands.
In 1995 he presented with acute urinary retention: cystoscopy was normal. From 1996 he complained of calf muscle pain and weakness, symptoms which were exacerbated by exercise. This progressively worsened and neurological assessment in 1998 identified a spastic diplegia associated with reduced sensation to vibration, light touch and pinprick. Serological tests for syphilis and borelia were negative. Serum folate and B12 levels were normal. Cerebrospinal fluid showed no oligoclonal bands and magnetic resonance imaging of spine was normal. Nerve conduction studies revealed a demyelinating motor neuropathy. The diagnosis of adrenomyeloneuropathy was confirmed by raised circulating concentrations of very long chain fatty acids (VLCFA) (Table 1). The neuropathic pain increased and he developed myoclonus. In 2002 he was wheelchair bound with an indwelling urinary catheter. Subsequently he has developed features of a dementia of frontal lobe type.
Table 1.
Very long chain fatty acid values and clinical features of affected family members
| Case | II-4 | II-3 | II-2 | IV-1 | IV-2 | Normal values (μmol/L) |
|---|---|---|---|---|---|---|
| C26 | 2.15 | 2.21 | 4.48 | 3.43 | 2.98 | 0.33-1.39 |
| C26:C22 ratio | 0.055 | 0.059 | 0.066 | 0.070 | 0.061 | <0.030 |
| C24:C22 ratio | 1.49 | 1.12 | 1.40 | 1.33 | 1.27 | 0.32-1.07 |
| Age at onset (years) | 31 | 38 | 40 | 2.5 | 2.0 | |
| Adrenal failure | Yes | Yes | Yes | Yes | Yes | |
| Cerebral involvement (symptomatic) | Yes | No | No | Yes | No |
Case II-3
The index case had three brothers (Figure 1) who were invited for assessment after the first diagnosis was made. His twin brother (non-identical) was seen at age 39 and gave an 18 month history of paraesthesiae in both legs but no weakness. He had no abnormal pigmentation or postural drop of blood pressure. Muscle tone was increased with brisk reflexes on the left. Sensation of light touch and pinprick was impaired on the soles of both feet. A short synacthen test showed cortisol 258 nmo/l at baseline, rising to 323 nmol/l at 30 min and 386 nmol/l at 60 min. Glucocorticoid replacement therapy was started. There has been no further clinically evident neurological deterioration on follow up.
Case II-2
This older brother described paraesthesiae in both legs. For 2 years he had been occasionally stumbling or tripping and his legs sometimes felt stiff. He complained of dizziness on standing and was easily tired. There was no abnormal pigmentation or postural drop in blood pressure.
Reflexes were brisk with left ankle clonus demonstrable. He had lost sensation to vibration, light touch and pin prick up to the knees. A short synacthen test was abnormal, with cortisol 300 nmol/L at baseline, 266 at 30 min and 289 at 60 min. Glucocorticoid replacement was initiated. This patient underwent formal neuropsychological and neurophysiological testing. Intellectual and memory functions were within the normal range. Somatosensory evoked potentials showed mild delay in latency in tibial and median nerves suggestive of delay in central transmission of impulses through spinal cord and brain. Brainstem auditory evoked potentials were not clearly abnormal. Visual evoked potentials showed abnormal latency in the left eye. The overall picture was of mild central demyelination. There has been no clinically evident neurological deterioration on follow up.
Case II-1
The patient chose not to have any investigations as he had only two sons who could not be affected.
Childhood-onset adrenoleukodystrophy: Case IV-1
The first affected grandchild (Figure 1) was born in 1997. He was tested electively in 2000 after the diagnosis of adrenomyeloneuropathy was made in his maternal grandfather. At this time he was asymptomatic.
Increased VLCFA (very long-chain fatty acids) were found and the presence of the same genetic mutation (P543L) was confirmed. In 2001 he had an abnormal short synacthen test and was commenced on glucocorticoid replacement. His baseline cortisol was 246nmol/L. After 250 g of synacthen, his 30 min cortisol was 284 nmol/L, with a 60 min result of 282 nmol/L. At the age of 2½ years formal assessment of speech and language confirmed developmental delay. His motor development was within normal limits. Visual assessment at age 3 revealed a right convergent squint but nothing else. Visual evoked potentials were normal 15 months later. Weight and height were within the normal range for his age.
From age 61/2 it was noted that speech and language development was becoming more delayed with increasing behavioural problems. Regression of gross motor function was noted with increasing dyspraxia. Formal neurological examination was otherwise normal. Assessment by an educational psychologist led to placement in a special school. In 2004 a magnetic resonance imaging scan of the brain showed some early abnormalities. In light of this, and evidence of learning difficulties, he underwent bone marrow transplantation, but subsequently developed graft versus host disease and died.
Case IV-2
The younger affected grandchild was born in 2001. An antenatal diagnosis was made at 10 weeks gestation on chorionic villus sampling. At the time the parents were uncertain as to whether the pregnancy would be continued if positive. In March 2003 a short synacthen test was abnormal and he has required regular glucocorticoid replacement. Salivary cortisols showed values of 165 nmol/L at baseline, 150 nmol/L at 10 min, 135nmol/L at 20 min, 120 nmol/L at 30 min and 150 nmol/L at 40 min. At last assessment aged 3, growth and development were normal, with no abnormal neurological signs. Visual evoked potentials were also normal. Magnetic resonance imaging of brain in 2004 was normal.
The family have been advised by clinical genetics services. This child has also been tissue typed in case of neurological deterioration at which point bone marrow transplantation may be indicated.
Biochemistry
The VLCFA results are given in Table 1.
Molecular genetics
Direct sequencing of both strands of an exon 6 PCR product identified the mutation P543L. This gene variant introduces a novel Pst 1 site (there is another Pst 1 site in exon 6 of the gene which acts as an internal control for digestion). The P543L mutation was shown to be present in each of the 3 brothers (cases II-2, II-3 and II-4) and the 2 grandchildren (cases IV-1 and IV-2) described above. At the time we first identified this mutation it had not been reported elsewhere in the literature.
COMMENT
Pathology
Adrenomyeloneuropathy/adrenoleukodystrophy is an X-linked recessive disorder characterized by a defect in β-oxidation of VLCFA (>22 carbon atoms) in peroxisomes. There is secondary neuroinflammatory change with demyelination of brain and spinal cord. The nervous system manifestations are of two distinct categories: adrenomyeloneuropathy and adrenoleukodystrophy.3 Adrenoleukodystrophy is an inflammatory myelinopathy that usually begins in the parieto-occipital regions and is often rapidly progressive. The classic form usually has onset in childhood (childhood cerebral adrenoleukodystrophy), with rapid neurological deterioration leading to a vegetative state, although the condition may also become manifest in adulthood (adult onset cerebral adrenoleukodystrophy). In contrast, adrenomyeloneuropathy is a non-inflammatory distal axonopathy involving mainly the spinal cord, long tracts and to a lesser extent peripheral nerves. There is a slowly progressive diplegia and a peripheral neuropathy with or without primary adrenal failure. Primary testicular failure, red-green colour blindness and depression may also be seen. The disorder affects 1 in 20-100 000 males most commonly as cerebral adrenoleukodystrophy in childhood or adrenomyeloneuropathy in adulthood.3,4
Biochemistry
The gene in question (ABCD1) codes for a peroxisomal membrane protein, adrenoleukodystrophy protein (ADLP), which is a member of the adenosine triphosphate binding cassette (ABC) transporter superfamily.3 Saturated VLCFA such as tetracosanoic (C24:0) and hexacosanoic acid (C26:0) accumulate in brain, adrenal glands and plasma.5 This accumulation is associated with failure of peroxisomal very long chain fatty acyl CoA synthetase to initiate degradation by beta-oxidation. The abnormally high levels of VLCFA impair the stability of biological membranes.6 The abnormal gene is located in the Xq28 region. It consists of approximately 26 kilobases of DNA and encodes an mRNA of 4.3 kb and a protein of 745 amino acids.3 Over 500 mutations have been identified.1 Of these, 59% are missense mutations, 23% frame shifts, 10% are nonsense mutations, 5% are in-frame deletions-insertions and 4% are large deletions.6 The website [www.x-ald.nl] updates the mutations identified worldwide. The mutation identified here was first reported in an Italian family and subsequently in three Spanish families.7,8 It has been shown to inhibit expression of ADLP in cultured fibroblasts from affected patients.8
Clinical presentation
The adult onset form of the disease itself shows a wide range of phenotypic variability ranging from pure adrenomyeloneuropathy with neurological signs that are confined to the spinal cord and peripheral nerves to forms with variable cerebral involvement. Pure myelopathy, cerebral involvement or adrenal failure may occur alone. The mutation described here predisposes to both adrenoleukodystrophy and adrenomyeloneuropathy.
A single mutation may cause different phenotypes not only in unrelated patients but also within the same family.1,5 The reason for the phenotypic variability and variable age of presentation of adrenomyeloneuropathy/adrenoleukodystrophy is unknown. Modifier genes1,3,4 or environmental factors have been suggested.9
An Italian series of cases of idiopathic primary adrenal failure found 4/19 male patients (21%) who developed adrenal failure aged 14-36 years had adrenomyeloneuropathy/adrenoleukodystrophy.2 Of these four, two had no neurological signs 6-29 years after the diagnosis of adrenal failure. But magnetic resonance imaging showed cerebral involvement or spinal atrophy in all four and electrophysiological studies were abnormal. In boys with primary adrenal failure 50% of cases are due to adrenoleukodystrophy. In patients known to have adrenoleukodystrophy/adrenomyeloneuropathy, 90% of male children and 65% of adults will have impaired adrenal function. Abnormalities on examination or investigation, usually adrenomyeloneuropathy, can be found in 50% of female carriers.1
Diagnosis
Features suggestive of the diagnosis of adrenomyeloneuropathy/adrenoleukodystrophy are:
primary adrenal failure in a young male
a family history of more than one case of `multiple sclerosis'
a male relative with spinal cord disease
memory loss with failure to perform well at school or `attention deficit disorder'
idiopathic seizure disorder in childhood.
Adult patients should be assessed for long tract signs. These may be subtle or absent. Measurement of VLCFA levels is diagnostic of adrenomyeloneuropathy in adults. Other peroxisomal disorders may elevate levels in the young (e.g. Zellweger's syndrome, infantile Refsum's disease, neonatal adrenoleukodystrophy). Such a diagnosis has implications for the family and prenatal testing can be offered after appropriate genetic counselling. Molecular genetic analysis is recommended and in the UK is available from the Molecular Genetics Laboratory at Liverpool Women's Hospital. In the Spanish series, study of 62 affected families revealed 53 different mutations.8 In three patients (5%) no mutation was identified despite raised VLCFA and negative ADLP expression.
Management
Includes surveillance of neurological and endocrine function. Magnetic resonance imaging scans and neuropsychological testing may be used for monitoring. No clearly effective treatment based on influencing lipid metabolism has yet emerged although Lorenzo's oil (4:1 glyceryl trioleate and glyceryl trierucate) administered before the age of 6 may reduce the probability of developing neurological deficit in later life.1 Statins reduce circulating levels of VLCFAs but have not yet been shown to influence neurological or endocrine function. Bone marrow transplantation is an option in boys with early neurological features, abnormal magnetic resonance imaging scans and neuropsychological dysfunction but is not recommended in the severely affected group (i.e. performance IQ<80) and has a significant morbidity and mortality.10 Survival data are superior to those not transplanted with improvement in neurological function, magnetic resonance imaging scans and neuropsychological measurements.11 Patients up to the age of 20 have been transplanted.12
Competing interests None.
References
- 1.Moser HW, Dubey P, Fatemi A. Progress in X-linked adrenoleukodystrophy. Curr Opin Neurol 2004;17: 263-9 [DOI] [PubMed] [Google Scholar]
- 2.Laureti S, Aubourg P, Calcinaro F, et al. Etiological diagnosis of primary adrenal insufficiency using an original flowchart of immune and biochemical markers. J Clin Endocrinol Metab 1999;83: 3163-8 [DOI] [PubMed] [Google Scholar]
- 3.Moser HW. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain 1997;120: 1485-508 [DOI] [PubMed] [Google Scholar]
- 4.Moser HW, Smith KD, Watkins PA, Powers J, Moser AB. X-linked adrenoleukodystrophy. The Metabolic and Molecular Basis of Inherited Disease, 8th edn. New York: McGraw Hill, 2005: 3257-301
- 5.Moser AB, Kreiter N, Bezman L, et al. Plasma very long chain fatty acids in 3000 peroxisome disease patients and 29 000 controls. Ann Neurol 1999;45: 100-10 [DOI] [PubMed] [Google Scholar]
- 6.Kemp S, Pujol A, Waterman HR, et al. ABCD1 mutations and the X-linked adrenoleukodystrophy mutation database: role in diagnosis and clinical correlations. Hum Mutat. 2001:18; 400-515 [DOI] [PubMed] [Google Scholar]
- 7.Lira MG, Mottes M, Pignatti PF, et al. Detection of mutations in the ALD gene (ABCD1) in seven Italian families: description of four novel mutations. Human Mutat 2000;16: 271. [DOI] [PubMed] [Google Scholar]
- 8.Coll MJ, Palau N, Camps C, et al. X-linked adrenoleukodystrophy in Spain. Identification of 26 novel mutations in the ABCD1 gene in 80 patients. Improvement of genetic counselling in 162 relative females. Clin Genet 2005;67: 418-24 [DOI] [PubMed] [Google Scholar]
- 9.Berger J, Molzer B, Fae I, Bernheimer H. X-linked adrenoleukodystrophy (ALD): a novel mutation of the ALD gene in 6 members of a family presenting with 5 different phenotypes. Biochem Biophys Res Commun 1994;205: 1638-43 [DOI] [PubMed] [Google Scholar]
- 10.Krivit W, Lockman LA, Watkins PA, Hirsch J, Shapiro EG. The future for treatment by bone marrow transplantation for adrenoleukodystrophy, metachromatic leukodystrophy, globoid cell leukodystrophy and Hurler syndrome. J Inherit Metab Dis: 1995;18: 398-412 [DOI] [PubMed] [Google Scholar]
- 11.Auborg P, Blanche S, Jambaque I, et al. Reversal of early neurological and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. N Engl J Med 1990;322: 1860-6 [DOI] [PubMed] [Google Scholar]
- 12.Hitomi T, Mezaki T, Tsuji T, et al. Improvement of central motor conduction after bone marrow transplantation in adrenoleukodystrophy. J Neurol Neurosurg Psychiatry 2003;74: 373-5 [DOI] [PMC free article] [PubMed] [Google Scholar]